Question
Question: $\text{CH}_3\text{-CH=CH}_2 \xrightarrow[\text{peroxide}]{\text{HI}}$ The major product of the abov...
CH3-CH=CH2HIperoxide
The major product of the above reaction is,
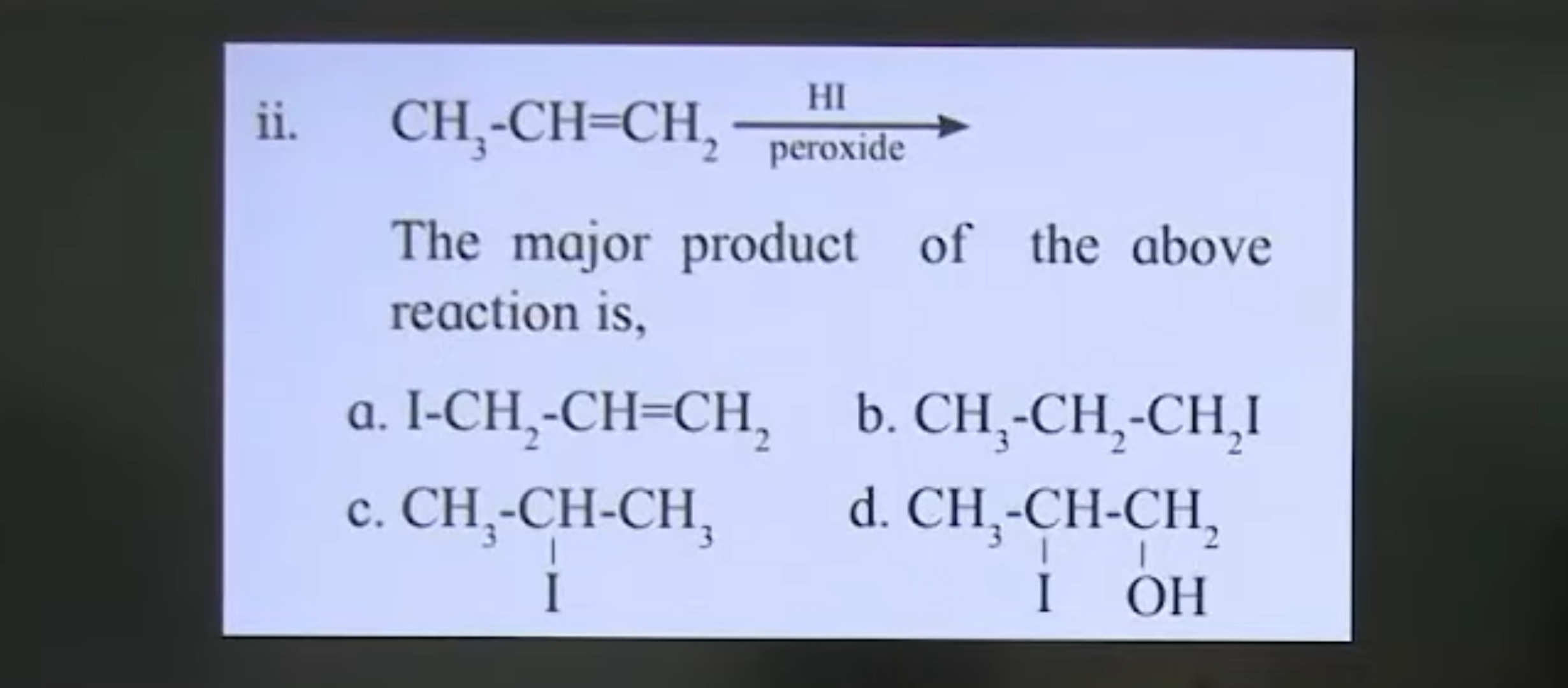
I-CH2-CH=CH2
CH3-CH2-CH2I
CH3-CH-CH3I
CH3-CH-CH2I OH
c
Solution
The reaction involves the addition of HI to propene (CH3-CH=CH2) in the presence of peroxide.
The peroxide effect (anti-Markovnikov addition) is observed only for HBr. It is not significant for HI because the radical chain propagation steps are energetically unfavorable (endothermic) and iodine radicals tend to dimerize.
Therefore, the addition of HI to propene will follow Markovnikov's rule. According to Markovnikov's rule, the hydrogen of HI adds to the carbon of the double bond with more hydrogens (CH2), and the iodine adds to the carbon with fewer hydrogens (CH).
CH3-CH=CH2+HIperoxideCH3-CHI-CH3
The major product is 2-iodopropane.
