Question
Question: In the following acid-base reaction, in which can backward reaction if favoured?...
In the following acid-base reaction, in which can backward reaction if favoured?
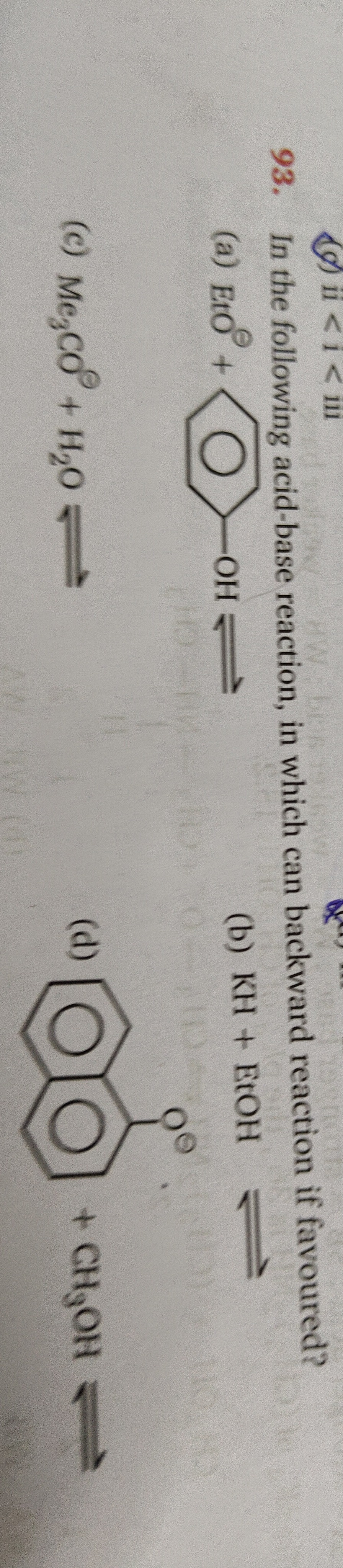
A
Eto+OH
B
KH + EtOH
C
Me3CO + H2O
D
- CH3OH
Answer
Me3CO + H2O
Explanation
Solution
The backward reaction is favored when the acid formed on the product side is weaker than the acid on the reactant side. This corresponds to the weaker acid being on the product side.
(a) Phenol (pKa ≈ 10) is a stronger acid than Ethanol (pKa ≈ 16-18). Forward reaction favored. (b) Ethanol (pKa ≈ 16-18) is a stronger acid than H₂ (pKa ≈ 35). Forward reaction favored. (c) Water (pKa ≈ 14) is a stronger acid than tert-Butanol (pKa ≈ 18). Backward reaction favored. (d) Methanol (pKa ≈ 15.5) is a stronger acid than Naphthol (pKa ≈ 9.5-10). Forward reaction favored.
Thus, the backward reaction is favored in option (c).
