Question
Question: The provided image shows the chemical structure of a compound. The question is not explicitly stated...
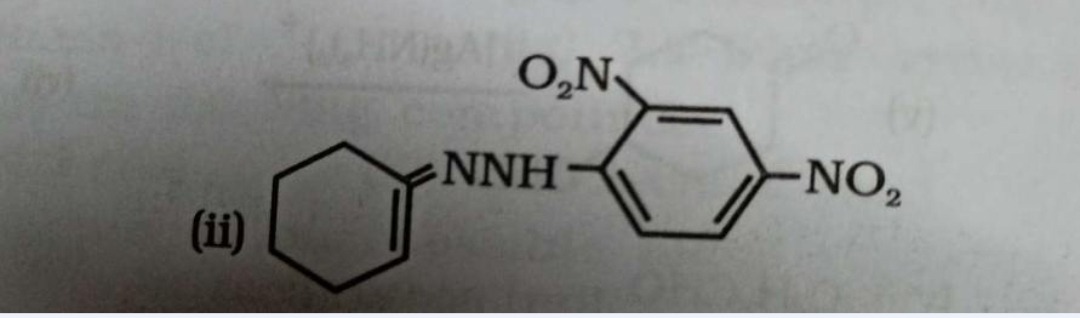
The provided image shows the chemical structure of a compound. The question is not explicitly stated, but based on the format and common JEE/NEET problems, it is likely asking for the identification, classification, or properties of the compound.
The structure consists of three parts connected in series:
- Cyclohex-1-en-1-yl group: This is a six-membered cyclic alkene. The ring has a double bond, and the attachment to the rest of the molecule is at one of the carbons of the double bond. Specifically, it is a cyclohexenyl group where the double bond is within the ring, and the attachment is to a carbon that is part of this double bond.
- Hydrazine linkage: This is the -NH-NH- group, which connects the cyclohexenyl group and the phenyl group.
- 2,4-Dinitrophenyl group: This is a benzene ring substituted with two nitro groups (-NO2) at the ortho (position 2) and para (position 4) positions relative to the point of attachment to the hydrazine linkage.
Combining these parts, the compound is N-(cyclohex-1-en-1-yl)-N'-(2,4-dinitrophenyl)hydrazine.
This compound is a substituted hydrazine. Hydrazines are derivatives of ammonia where one or more hydrogen atoms are replaced by amino groups. In this case, both hydrogen atoms on one nitrogen of hydrazine are replaced by an organic group (cyclohex-1-en-1-yl), and one hydrogen on the other nitrogen is replaced by another organic group (2,4-dinitrophenyl). Since the two organic groups attached to the nitrogen atoms are different, it is an unsymmetrical substituted hydrazine.
Key Features:
- Functional Groups: Hydrazine (-NH-NH-), alkene (C=C in cyclohexene), nitro groups (-NO2).
- Reactivity:
- The hydrazine moiety can react with aldehydes and ketones to form hydrazones. The nitrogen atom attached to the cyclohexenyl group is expected to be more nucleophilic than the one attached to the electron-deficient dinitrophenyl group, thus participating more readily in such reactions.
- The double bond in the cyclohexene ring can undergo addition reactions (e.g., halogenation, hydrogenation).
- The nitro groups are electron-withdrawing, making the phenyl ring electron-deficient and less susceptible to electrophilic aromatic substitution. They also increase the acidity of the adjacent NH proton.
Nomenclature: The systematic IUPAC name is N-(cyclohex-1-en-1-yl)-N'-(2,4-dinitrophenyl)hydrazine.
Classification: Substituted hydrazine.
N-(cyclohex-1-en-1-yl)-N'-(2,4-dinitrophenyl)hydrazine
Solution
The compound is identified as a substituted hydrazine, specifically N-(cyclohex-1-en-1-yl)-N'-(2,4-dinitrophenyl)hydrazine, by analyzing its structural components: a cyclohexenyl group, a hydrazine linkage, and a 2,4-dinitrophenyl group.
