Question
Question: Initially, flask A contained oxygen gas at 27°C and 950 mm of Hg, and flask B contained neon gas at ...
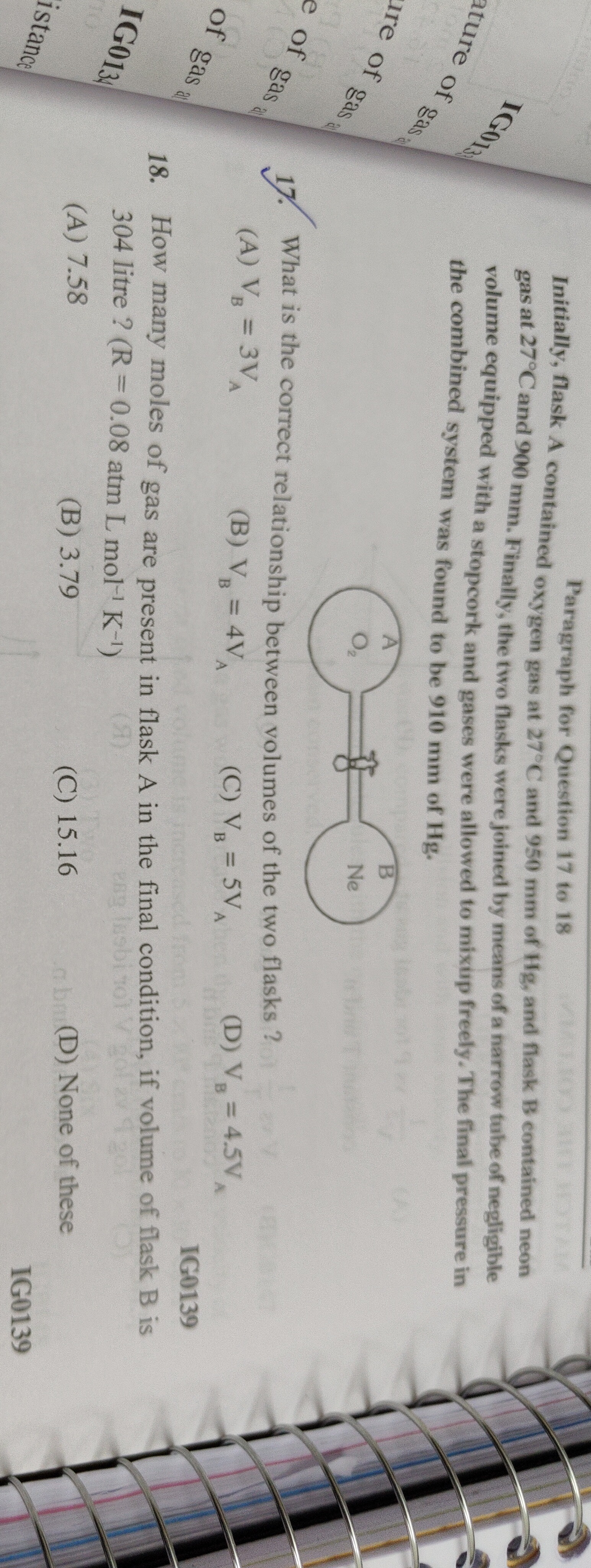
Initially, flask A contained oxygen gas at 27°C and 950 mm of Hg, and flask B contained neon gas at 27°C and 900 mm. Finally, the two flasks were joined by means of a narrow tube of negligible volume equipped with a stopcork and gases were allowed to mixup freely. The final pressure in the combined system was found to be 910 mm of Hg.
What is the correct relationship between volumes of the two flasks ?
VB = 3VA
VB = 4VA
VB = 5VA
VB = 4.5VA
VB = 4VA
Solution
Let VA and VB be the volumes of flask A and B, respectively. According to Boyle's Law, the pressure of a gas is inversely proportional to its volume at constant temperature. When the gases mix, the final total pressure (Pfinal) is the sum of the partial pressures of each gas in the combined volume (VA+VB). The partial pressure of gas A in the final mixture is PA,final=VA+VBPA,initial×VA. The partial pressure of gas B in the final mixture is PB,final=VA+VBPB,initial×VB. By Dalton's Law of Partial Pressures, Pfinal=PA,final+PB,final.
Substituting the given values: 910=VA+VB950×VA+VA+VB900×VB Multiplying by (VA+VB): 910(VA+VB)=950VA+900VB 910VA+910VB=950VA+900VB Rearranging the terms: 910VB−900VB=950VA−910VA 20VB=40VA VB=2VA
Re-checking with the options, if we assume VB=4VA: 910(VA+4VA)=950VA+900(4VA) 910(5VA)=950VA+3600VA 4550VA=4550VA This confirms that VB=4VA is the correct relationship.
