Question
Question: If true enter1, else enter 0. A species with one or more unpaired electrons is called a free radic...
If true enter1, else enter 0.
A species with one or more unpaired electrons is called a free radical.
Solution
Homolytic cleavage i.e. the reaction which involves the breaking of the covalent bond in such a way that each atom separates with the electron of the shared pair and results in the formation of the neutral species i.e. the free radicals.
Complete step by step answer:
-A free radical is an atom or group of atoms having an odd or unpaired electron. It is denoted by putting a dot (·) against the symbol of the atom or group of atoms. E.g.· stands for chlorine free radicle etc.
-They are electrical neutral species which are usually formed by the homolytic cleavage (i.e. the cleavage in which the a covalent bond is equally shared between the two bonded atom and they gets separated with each atom having once electron of the covalent bond) of the molecules in reactions taking place in gas phase or in non-polar solvents. E.g.:
Cl—Cl → 2Cl·
Chlorine free radicals
-Due to the presence of unpaired electrons i.e. odd electrons, they are paramagnetic in nature. They are highly short- lived species. They are extremely reactive and as soon as they are formed, they immediately participate in the further reaction and their greater reactivity is due to the tendency of the odd-electron in them to get paired up.
Note: The free radicals which contain the carbon atom, their state of hybridization is not clearly known and it is indicated that most of alkyl free radicals such as methyl radicals are actually planar.
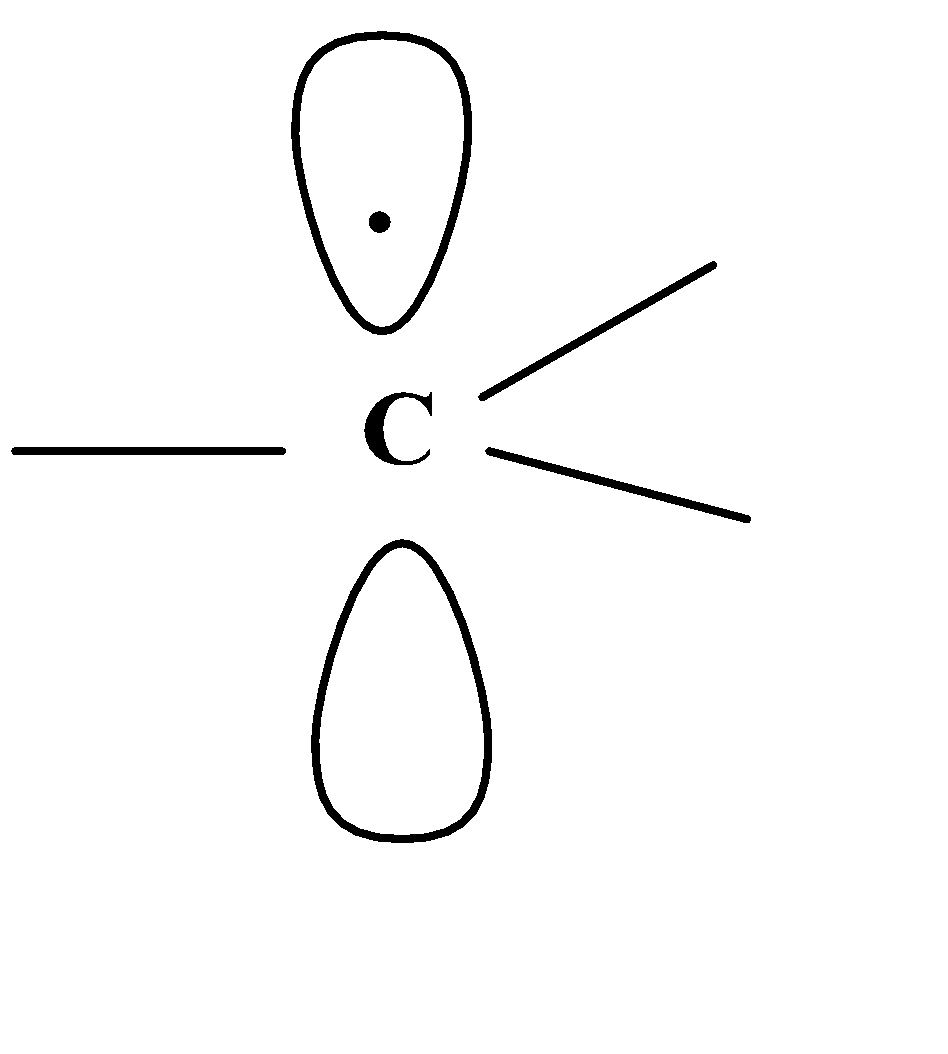
This means that carbon atom which carries odd electron has sp2 hybridization and the other three hybrid orbitals are involved in bond formation with other atoms and the unhybridized p- orbital is at the right angle to plane and carries the odd electron.
