Question
Question: If the radius of \[N{{a}^{+}}\]is 95 pm and that of \[C{{l}^{-}}\]is 181 pm, then: A. coordination...
If the radius of Na+is 95 pm and that of Cl−is 181 pm, then:
A. coordination number of Na+is 6
B.coordination number of Cl−is 8
C.length of the unit cell is 552 pm
D.length of the unit cell is 380 pm
Solution
Hint: Radius ratio is used to find out the coordination number of the ionic solids.
NaCl is an ionic solid with fcc (face-centred cubic) lattice. So, the edge length of the unit cell can be determined from the arrangement of the ions in the fcc lattice.
Complete step by step answer:
The radius ratio of the ionic solid is the ratio of the radius of the cation to the radius of the anion.
Formula of the radius ratio:
Radius ratio = radius of the anion(r−)radius of the cation(r+)
In an ionic solid, a cation is surrounded by the highest number of anions around it. Greater radius ratio indicates higher coordination number.
Here, the radius of the cation (r+) is 95 pm and the radius of the anion (r−) is 181 pm.
So, radius ratio = 18195 = 0.528
When the radius ratio lies between 0.414 and 0.732, the coordination number of the ions is 6.
So, the coordination number of Na+is 6.
fcc structure means there will be eight atoms at the eight corners of the cube and one atom at the centre on each of the six faces of the cube. The structure of the lattice is a as follows:
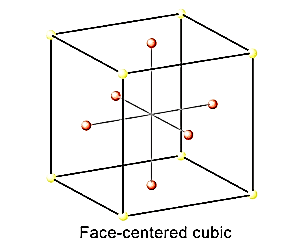
The radius of Na+is 95 pm and that of Cl−is 181 pm. Due to its fcc structure,
length of the unit cell = 2 ×distance between Na+and Cl−
= 2 ×(Na++Cl−)
= 2 ×(95+181) pm = 552 pm
So, the correct options are A and C.
Note: When the radius ratio lies between 0.225 and 0.414, the coordination number of the ions is 4. When the radius ratio lies between 0.414 and 0.732, the coordination number of the ions is 6.
