Question
Question: If the radius of an octahedral void is r and radius of atoms in close packing is R, the relations be...
If the radius of an octahedral void is r and radius of atoms in close packing is R, the relations between r and R is
A
r=0.414R
B
R=0.414r
C
r = 2R
D
r−2R
Answer
r=0.414R
Explanation
Solution
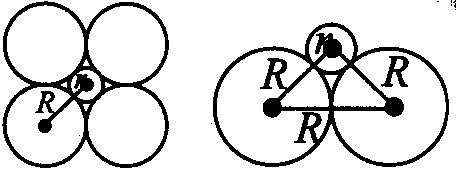
(2R)2=(R+r)2+(R+r)2
2R=R+r
(2−1)R=r
0.414R=r
