Question
Question: If the quantum numbers for the \[{5^{th}}\] electron in the carbon atom are \(2,1, - 1, + \dfrac{1}{...
If the quantum numbers for the 5th electron in the carbon atom are 2,1,−1,+21 , then for the 6th electron, these values would be:
A.1,1,0,−21
B.2,0,1,+21
C.2,1,1,−21
D.2,1,0,+21
Solution
We know that the value of n for p orbital is already given, we know that l=n−1 and total value of m=2l+1 where m has values ranging from –l to +l. The value of spin quantum number is +21 for clockwise rotation and −21 for anticlockwise rotation of electrons. Write the electronic configuration of carbon atoms and find these values.
Complete step by step answer:
Given, the quantum numbers for the 5th electron in the carbon atom are 2,1,−1,+21 and we have to find the values of all quantum numbers for 6th electron of carbon atom.
We know that carbon has atomic number 6 and the electronic configuration of electrons is 1s2,2s22p2
The representation of electrons in the orbital is given as-
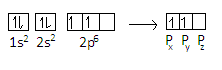
We can see from the representation that the 5th electron in the carbon atom is in 2px orbital and 6th electron of carbon atom is in2py.For fifth electron, the principal quantum number n=2 , azimuthal quantum number l=1 , magnetic quantum number m=−1 and spin quantum number s=+21 For the sixth electron, n=2and l=1 as the principal quantum and azimuthal quantum number will remain the same as the electron is in p=orbital.We know that total values of m=2l+1 and can have values from –l to +l.On putting value of l in the formula, we get-
⇒ m=2+1=3
So there will be three values of m ranging from −1to +1but since the fifth electron already has one value of m=1 so either m=0 or - 1 Since the direction of the electron is same as that of the fifth electron then the value of s will also be same .i.e. +21 So the values of the sixth electron’s quantum numbers are-2,1, - 1,+21 .
Hence the correct answer is option D.
Note:
Quantum numbers are a set of numbers that tell the position and energy of an electron in an atom. Each quantum number tells different points about the electron-
-The principal quantum number ‘n’ tells about the number of the principal shell in which the electron is present.
-Azimuthal quantum number gives the shape of an orbital and is equal to the total number angular nodes of an orbital.
-Magnetic quantum number tells about the orientation and the total number of orbitals of a subshell.
-Spin quantum number gives the direction of the spin of the electron in the orbital.
