Question
Question: If the products of ozonolysis are acetone and formaldehyde then what is the initial compound? (A)...
If the products of ozonolysis are acetone and formaldehyde then what is the initial compound?
(A) 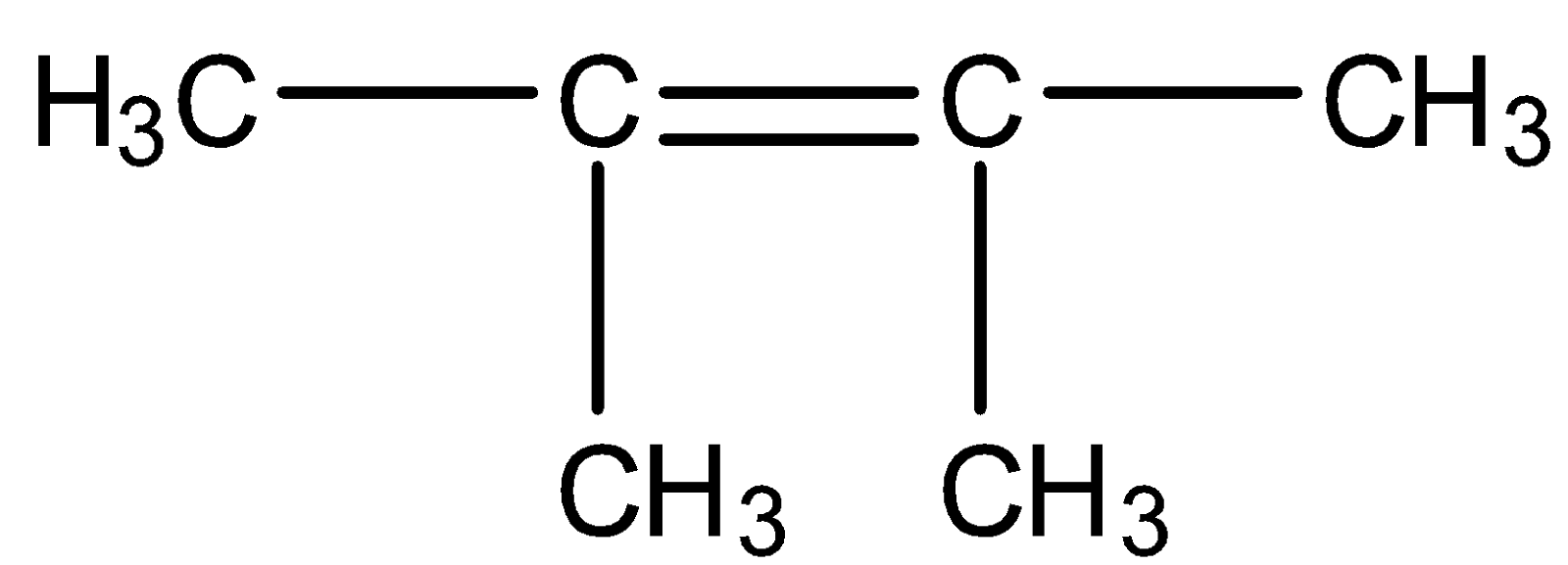
(B) 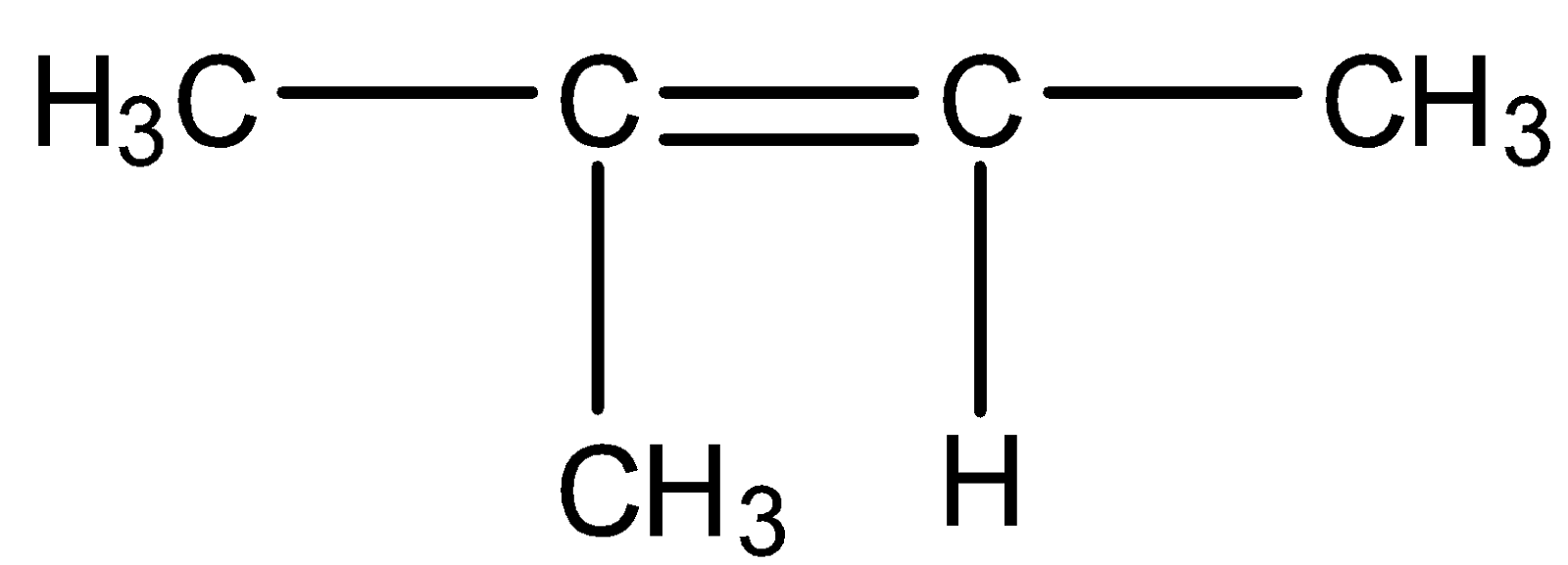
(C) 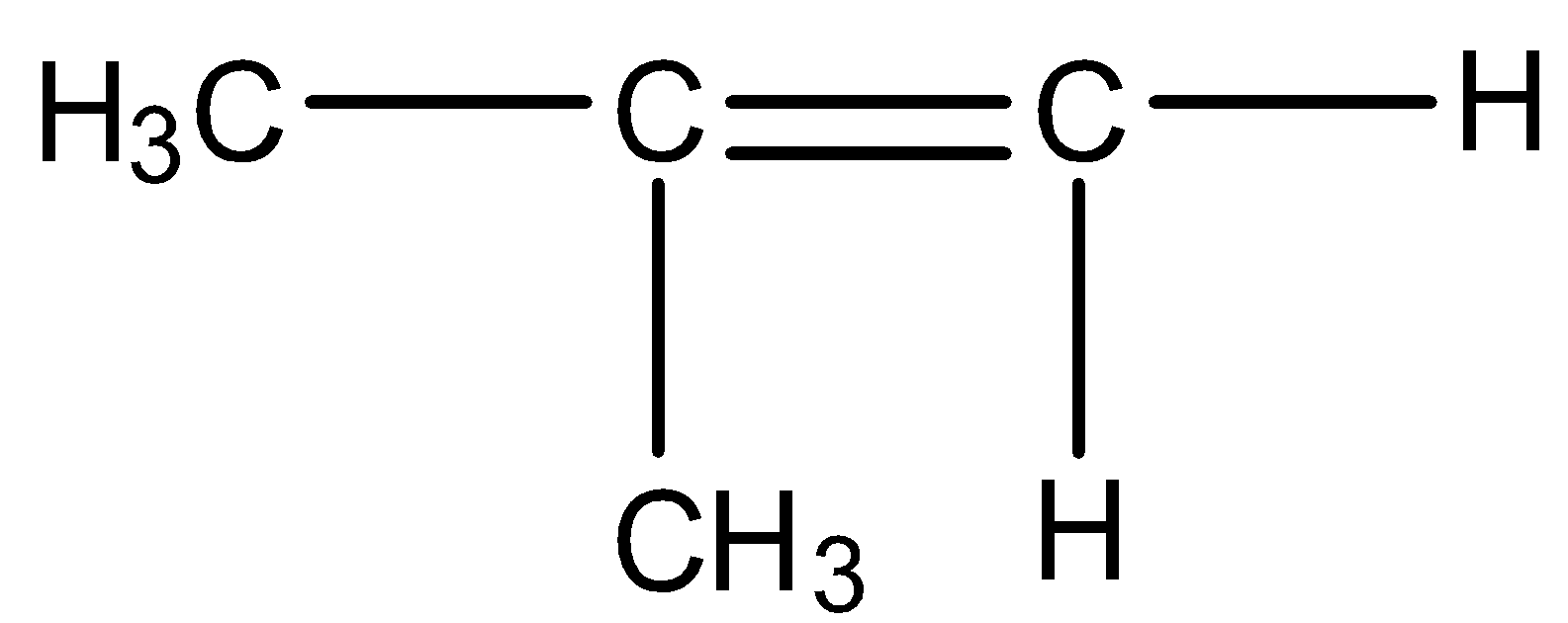
(D) 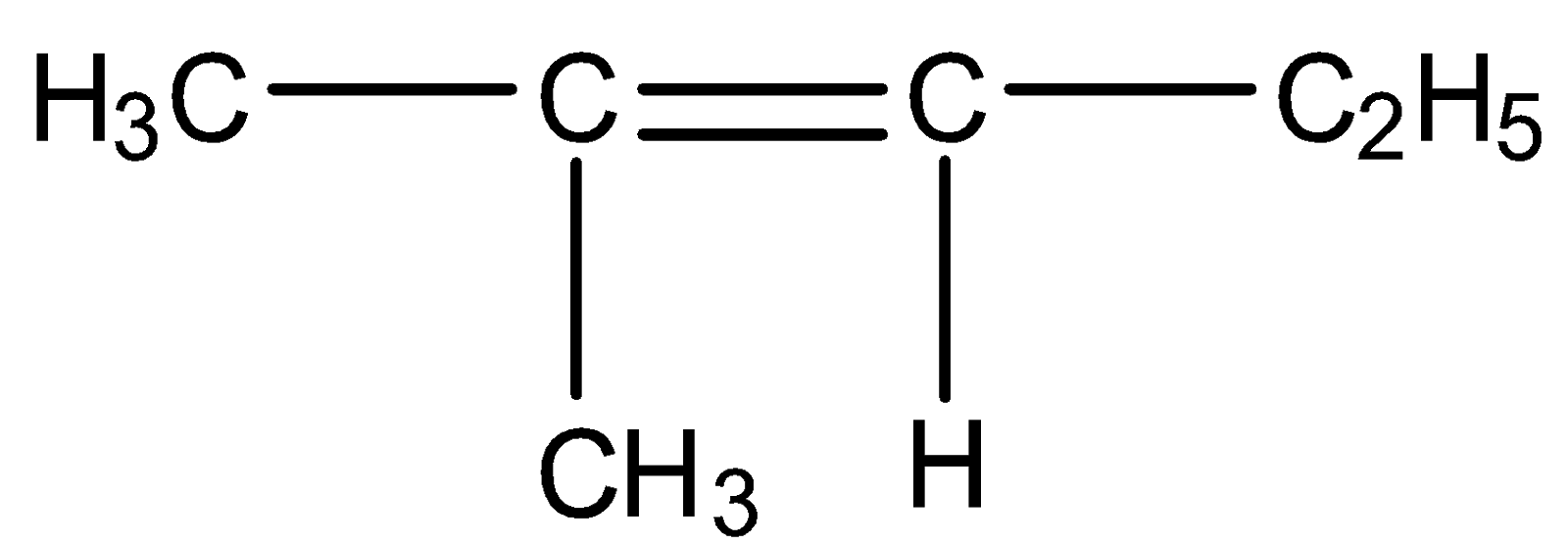
Solution
First we will know the mechanism of the ozonolysis as to what attacks and what is added to the substrate and what products are formed and what are the conditions. Then we will compare the structures in option to give the desired product.
Complete step by step answer:
Ozonolysis is a type of organic reaction in which there is addition of oxygen and nascent oxygen is also produced. The functional groups like alkene, alkyne, azo etc get a break from between and are replaced by double bonded oxygen to satisfy valency. Ozonolysis is mostly done on unsaturated substrates. It oxidizes the unsaturated compound to any carbonyl group example aldehydes, ketones, carboxylic acids etc.
Now we will see the mechanism of ozonolysis products formed.
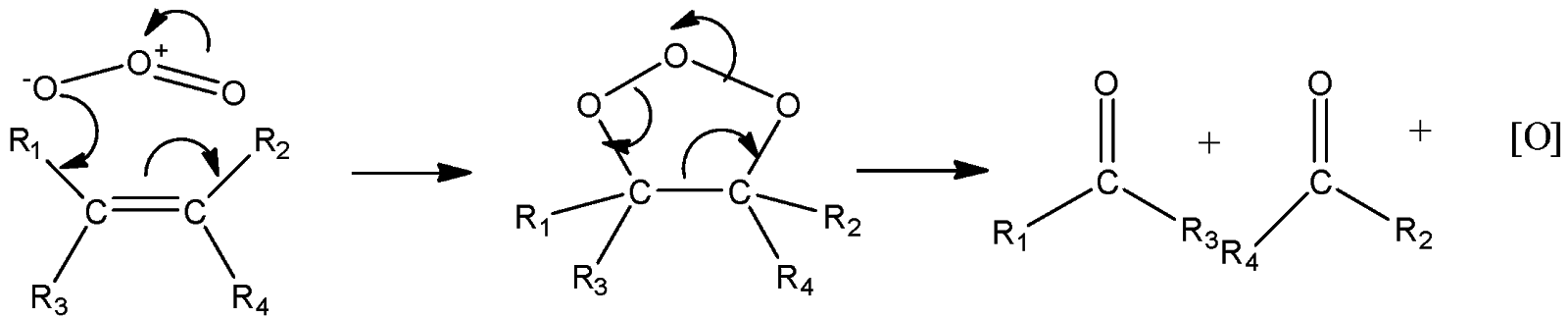
This is how we can analyze that the ketones formed above have groups attached in the products that were on the same carbon; no exchange or migration of groups happens; only the double bond is cleaved from between. So the shortcut for the product is to remove the double bond and replace it with double bonded oxygen in the form of carbonyl.
So we need our products to be acetone and formaldehyde. Acetone has two methyl groups and formaldehyde has two hydrogens on either side means we should have such reactants in which one carbon should have a methyl group and another carbon has two hydrogen groups. Such structure is present in option C
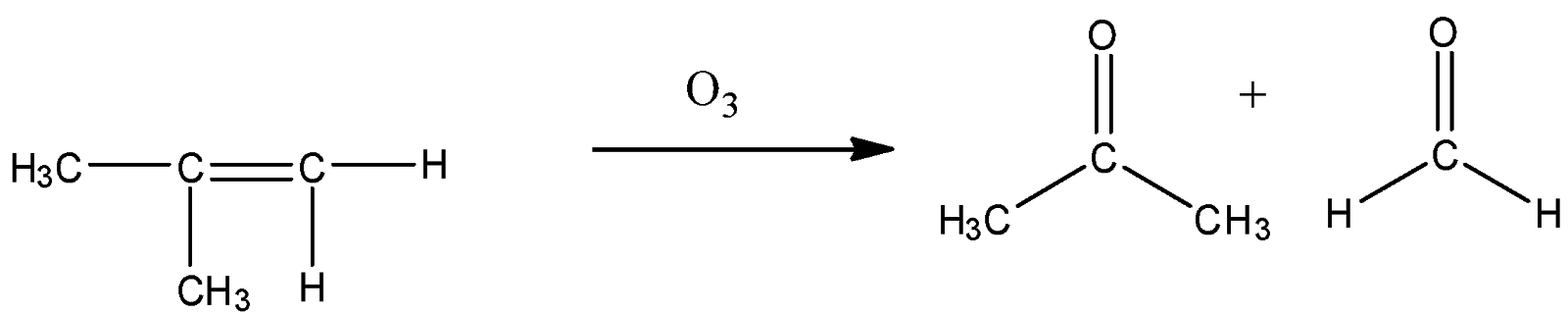
So, the correct answer is Option C.
Additional information:
In ozonolysis the intermediate form which is shown in the above mechanism is called ozonoid. This ozonoid forms into carbonyl compounds but due to nascent oxygen they again react and flips the carbonyl group by cyclo – addition.
Note: The ozonolysis is of two types oxidative ozonolysis and reductive ozonolysis. This depends on the reagent we are taking in combination to ozone, if we take zinc metal or dimethyl sulphide it will be reductive ozonolysis and when we use hydrogen peroxide then oxidative ozonolysis takes place and aldehyde is converted directly to carboxylic acid.
