Question
Question: If the principal quantum number n=6, the correct sequence of filling of electron will be: (A) \(ns...
If the principal quantum number n=6, the correct sequence of filling of electron will be:
(A) ns→(n−2) f→np→(n−1)d
(B) ns→(n−1) f→np→(n−2)d
(C) ns→np→(n−1)d→(n−2)f
(D) ns→(n−2)f→(n−1)d→np
Solution
Think about the Aufbau’s principle. Recollect what a principal quantum number is. Carefully take a look at the options and then choose the correct option. Take a look at the periodic table and think about the order in which the electrons will fill in the orbitals to get the answer.
Complete step by step solution:
- Principle quantum number (n) tells us the number of shells in which the last electron enters.
- Basically, the principal quantum number tells us about the valence shell.
- According to Aufbau’s rule, electrons will fill lower energy atomic levels first and then get filled up in the higher energy levels.
- Aufbau’s rule is represented below in the given diagram.
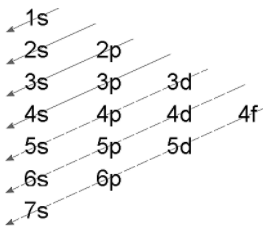
- The electrons will start filling up in the order:
1s then 2s then 2p then 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p and then 7s and 7p.
- 1s orbital has the least amount of energy.
- If the valence electron enters in the sixth orbital then, the electron enters in 6s orbital followed by 4f orbital then 5d orbital and then 6p orbital.
- Therefore, when n=6, the electron fills in the order 6s, 4f, 5d and 6p.
- So, the option ns→(n−2)f→(n−1)d→np is correct.
Therefore, option (D) is correct.
Note: Remember the electrons will start filling up from lower orbitals and then get filled in higher orbitals. This rule is known as Aufbau’s principle. The Principal quantum number is represented as ‘n’ which is the number of main shells in which electrons enter.
