Question
Chemistry Question on kinetics equations
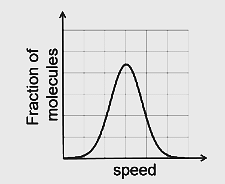
If the distribution of molecular speeds of a gas is as per the figure shown below, then the ratio of the most probable, the average, and the root mean square speeds, respectively, is
A
1:1:1
B
1:1:1.224
C
1:1.128:1.224
D
1:1.128:1
Answer
1:1:1.224
Explanation
Solution
In a symmetrical graph, the average value of speed corresponds to the midpoint, which is also the most probable speed.
However, when calculating the root mean square (rms) speed, we compute the average of the squares of the speeds and then take the square root. Since higher speeds contribute more to the squared value, the result will be higher than the average speed value.
So the Correct Option is (B): 1:1:1.224
