Question
Question: If the difference between the pressure inside and outside of a soap bubble is 6mm of water and its r...
If the difference between the pressure inside and outside of a soap bubble is 6mm of water and its radius is 8mm. What is the surface tension in dynes per cm?
A. 117.6
B. 256
C. 378
D. 450
Solution
The liquid surface will have a general tendency to shrink itself to the most minimum of the surface area, thus making the surface of the liquid behave like a stretched membrane. This phenomenon of the liquid is called the surface tension of the liquid. Water has a very high value of surface tension in general, compared to other liquids.
Complete step by step answer:
The formation of a bubble in the air can be explained by surface tension. Due to the surface tension of the soap bubble, it tries to occupy the minimum surface thereby, forming a circular bubble of soap.
However, there is the force acting on the bubble inside and outside due to pressure, and another force acting from the inside of the bubble due to stretching of the bubble because of surface tension.
Consider the soap bubble of radius R with all the forces acting on it
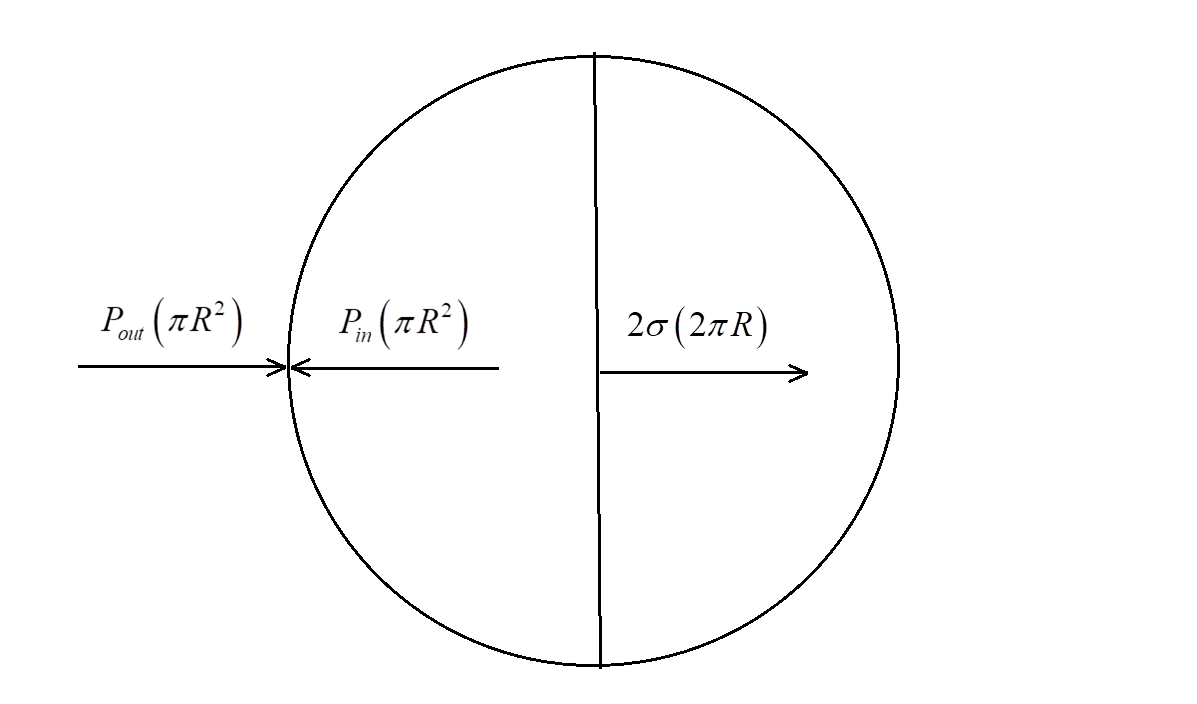
Here, there are 3 forces acting on the soap bubble as shown:
A. Force due to external pressure = Pout(πR2)∵F=P×A where A = area.
B. Force due to internal pressure = Pin(πR2)
C. Force due to surface tension of the bubble = 2σ(2πR) where σ= surface tension in dyne/cm.
Here, the 2πR represents the circumference of the soap bubble, and factor 2 is multiplied because the force acts on both the ends of the center.
By equating the forces above, we get
Pout(πR2)+2σ(2πR)=Pin(πR2)
Solving, we get –
⇒Pout(πR2)−Pin(πR2)=2σ(2πR) ⇒πR2(Pout−Pin)=4σπR ⇒Pout−Pin=πR24σπR
Difference in pressure, Pout−Pin=R4σ
In this problem, given difference in pressure, Pout−Pin=6mm=0.006m
We have to convert 6mm of water into standard unit pascal –
⇒Pout−Pin=0.006×1000×9.81 ⇒Pout−Pin=58.8Nm−2
Radius of the bubble, R=8mm=0.008m
Substituting in the expression for the difference in pressure, we can calculate the surface tension.
⇒Pout−Pin=R4σ
⇒58.8=0.0084σ
On solving,
⇒σ=458.8×0.008=40.4704=0.1176Nm−1
Converting it to dyne/cm, we have –
1Nm−1=1000dyne−cm−1
⇒σ=0.1176×1000=117.6 dyne/cm
∴ The surface tension is 0.1176×1000=117.6 dyne/cm. Therefore, the correct option is Option A.
Note:
There are two units of surface tension. The surface tension bears the dimension of force/length.
The SI unit is Newton per meter and the CGS unit is dyne per centimeter.
The dyne is the force unit in the CGS system which is defined as :
1 dyne = 1 g−cm.s−2
This is equal to –
1 dyne = 1 g−cm.s−2= 1×s210−3kg×10−2m=10−5kg−m.s−2
Since, 1N=1kg−ms−2,
1 dyne = 10−5N
And thus, the conversion formula for converting dyne/cm to N/m is –
1 dyne/cm = 10−2m10−5N=10−3Nm−1
