Question
Question: If phosphorus acid, tetrathionic acid and pyrophosphoric acid have a number of acidic hydrogen which...
If phosphorus acid, tetrathionic acid and pyrophosphoric acid have a number of acidic hydrogen which can be represented as x, y and z respectively. Then find the value of x+y+z?
Solution
We have to know that the acidic hydrogen is present in all acids and the number of hydrogens present in an acid tells us how the nature of that particular acid. To answer this question we must know the structure of acids given thus it is easy to answer.
Complete answer:
Let us start by studying phosphorus acid, the molecular formula is H3PO3 and its structure can be represented as,
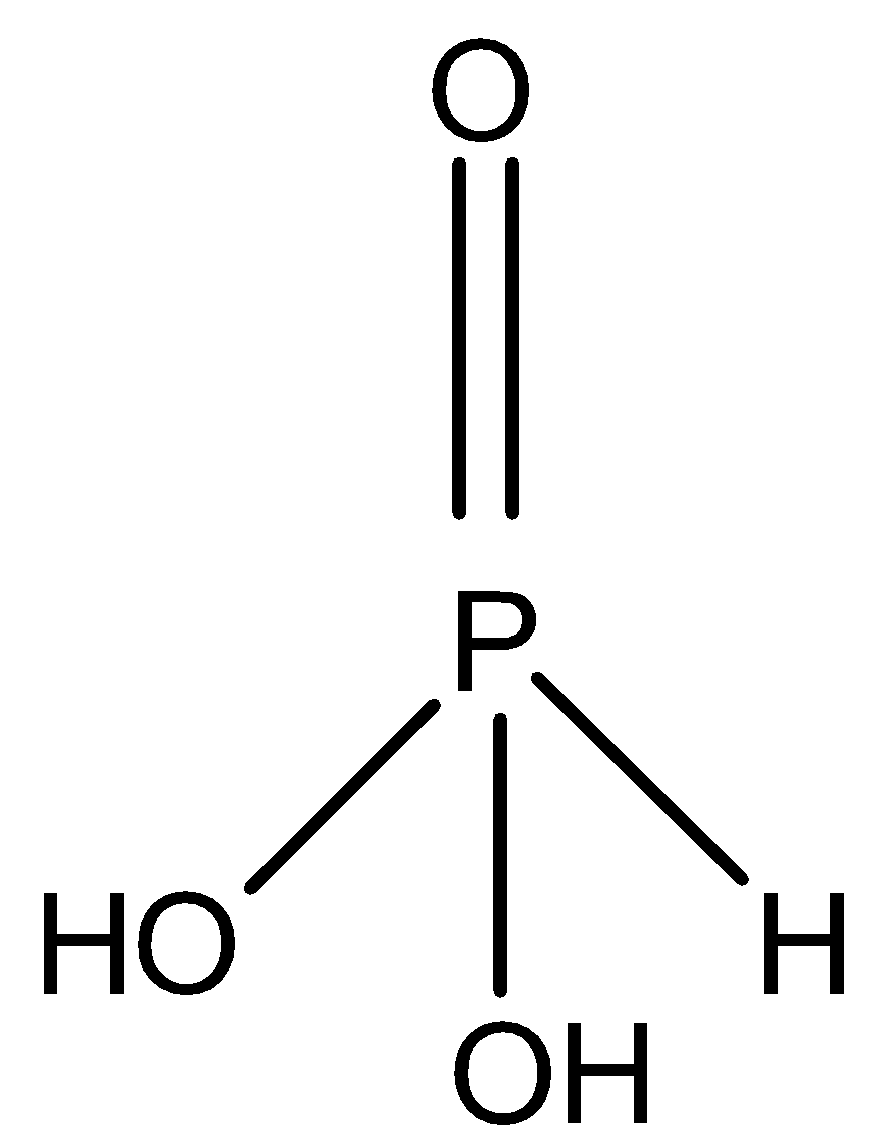
Phosphorus acid contains 2 hydroxyl groups i.e. O−H bond thus it is dibasic in nature, so acidic hydrogen is two thus value of x=2.
Second is tetrathionic acid, the molecular formula is H2S4O6 and its structure can be represented as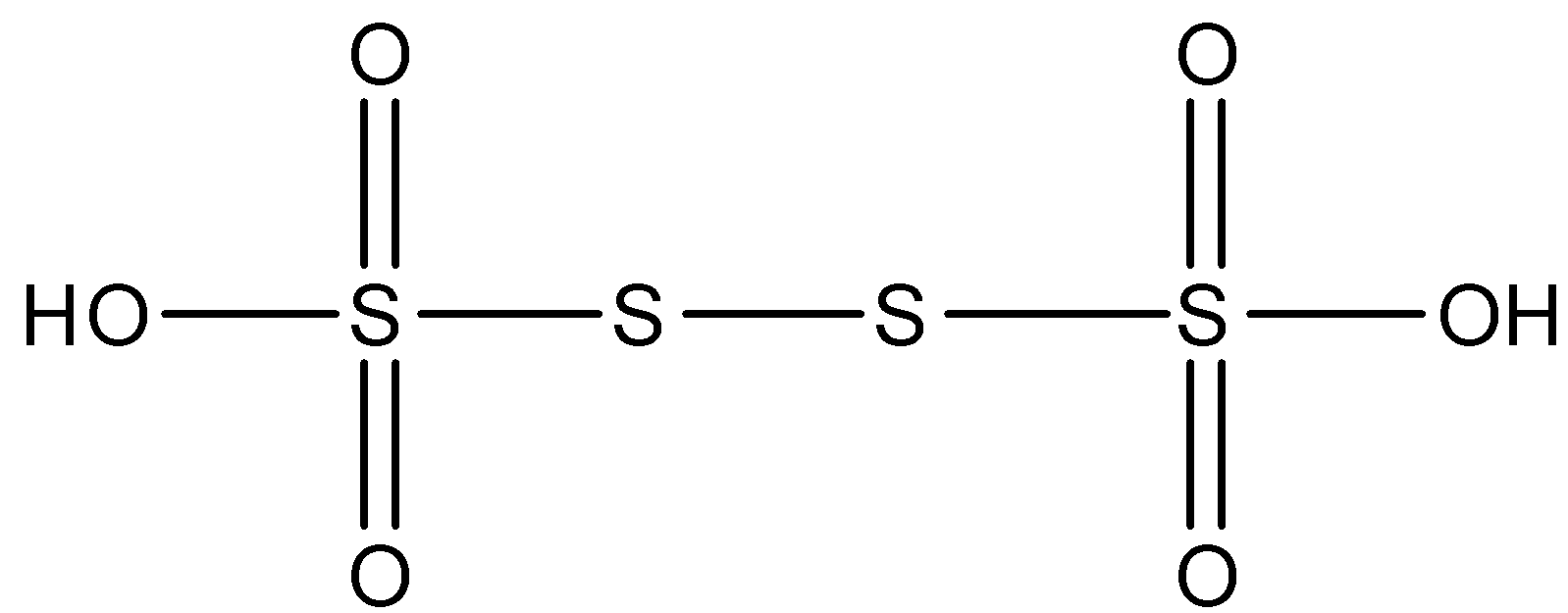
Tetrathionic acid contains 2 hydroxyl groups i.e. O−H bond thus it is dibasic in nature, so acidic hydrogen is two thus value of y=2.
The last is pyrophosphoric acid, the molecular formula is H4P2O7 and the structure can be represented as
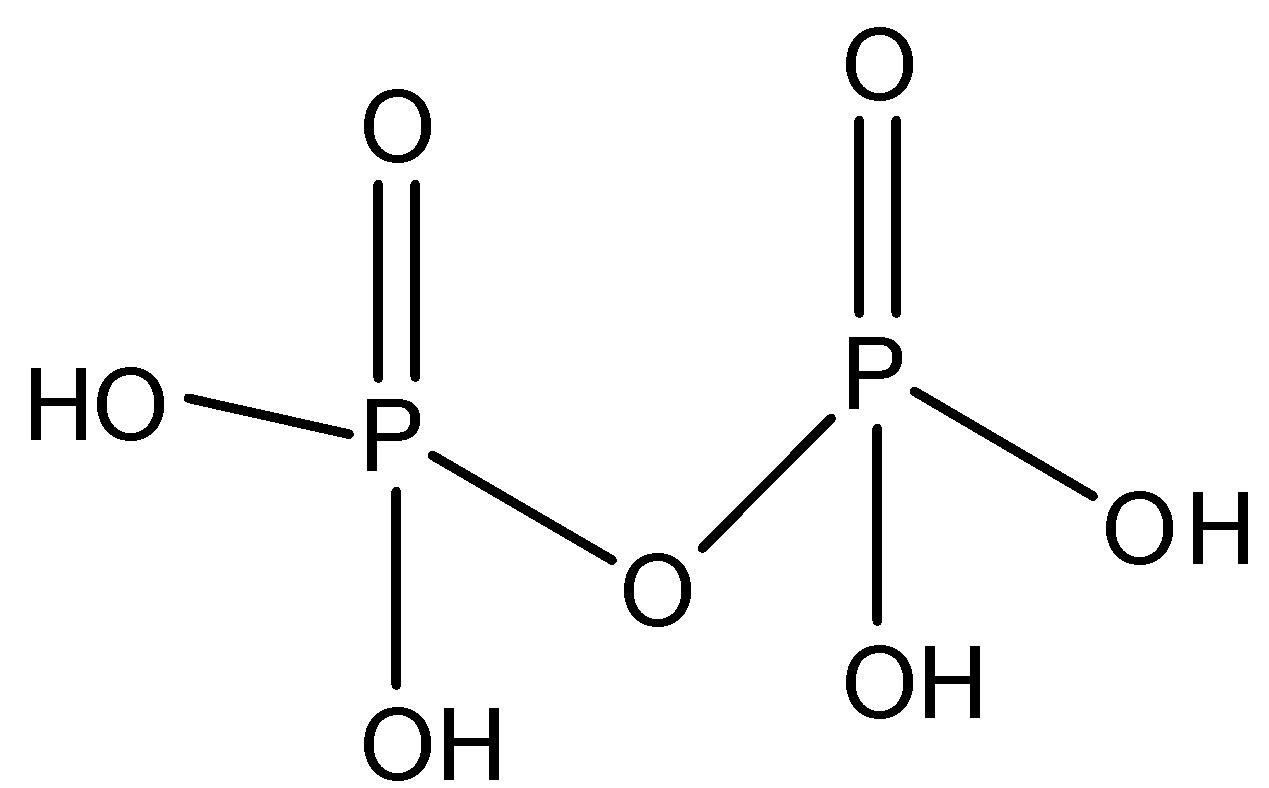
Pyrophosphoric acid contains 4 hydroxyl groups i.e. O−H bond thus it is dibasic in nature, so acidic hydrogen is two thus value of z=4.
