Question
Question: If “n” number of \[{{H}_{3}}P{{O}_{4}}\] molecules are polymerized to produce chain molecule and rin...
If “n” number of H3PO4 molecules are polymerized to produce chain molecule and ring molecule separately, then the number of P−O−P linkage forms is, respectively.
(A) n and (n-1)
(B) (n-1) and (n-1)
(C) (n-1) and n
(D) n and n
Solution
Polymerization is a method or a process in which monomer molecules react together through chemical reaction to form polymer chains or three-dimensional network structure.
Complete step by step answer:
H3PO4 means phosphoric acid is a monomer that undergoes polymerization forms two types of products.
One type of product is ring structure based molecule.
Second type is a chain type structure based molecule.
In case of a ring structure, the number of P−O−Plinkages will be one less (n-1) than the number of phosphorus atoms (P) because of the fact that each phosphorus atom is shared by two rings in the structure.
Though, in case of a chain structure there is no sharing and the number of P−O−P linkages is equal to the number of phosphorus atoms (n).
So, the “n-1” P−O−P linkages in case of ring structure.
“n”P−O−Plinkages in case of chain structure.
Therefore “n-1” linkages are present in ring structure and “n” linkages are present in chain structure produced by the polymerisation of phosphoric acid.
So, the correct option is A, n and (n-1).
Note: Don’t be confused in the given options A and C.
In chain structure “n”P−O−P linkages and in ring structure “(n-1)” P−O−P linkages.
Don’t be confused with ring structure and chain structure.
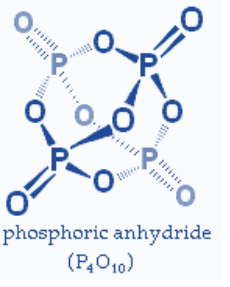
Ring structure
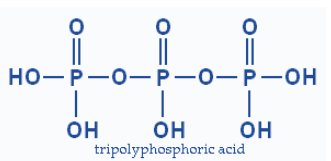
Chain structure
