Question
Question: If meta-nitroaniline is chlorinated, the major product is: (A) 
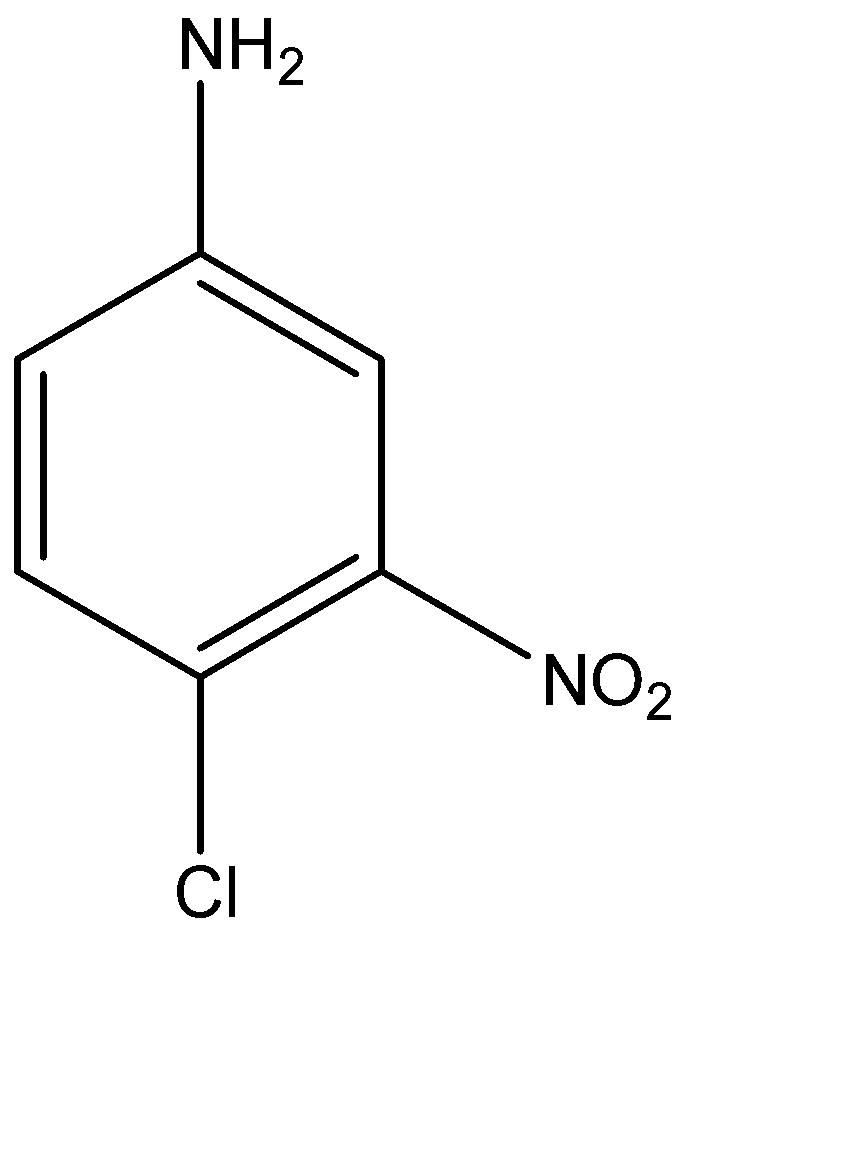
(B)
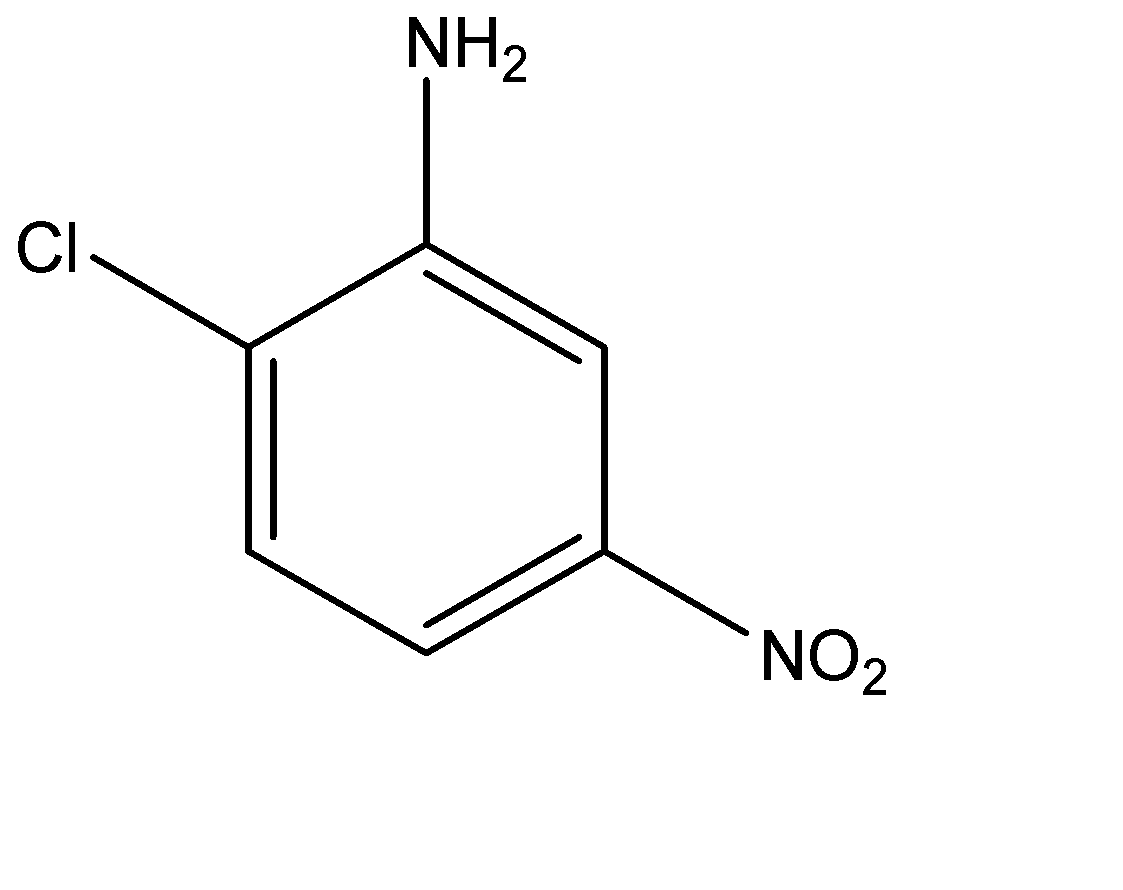
(C)
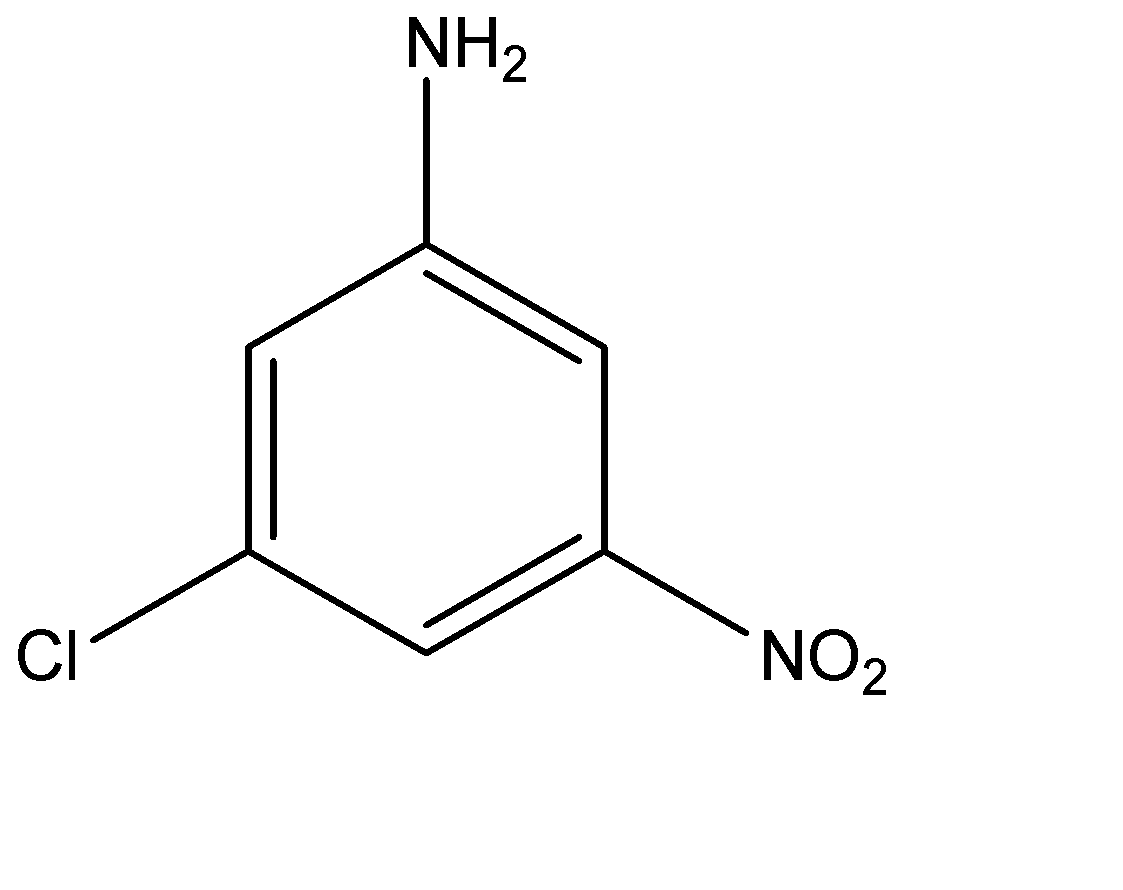
(D)
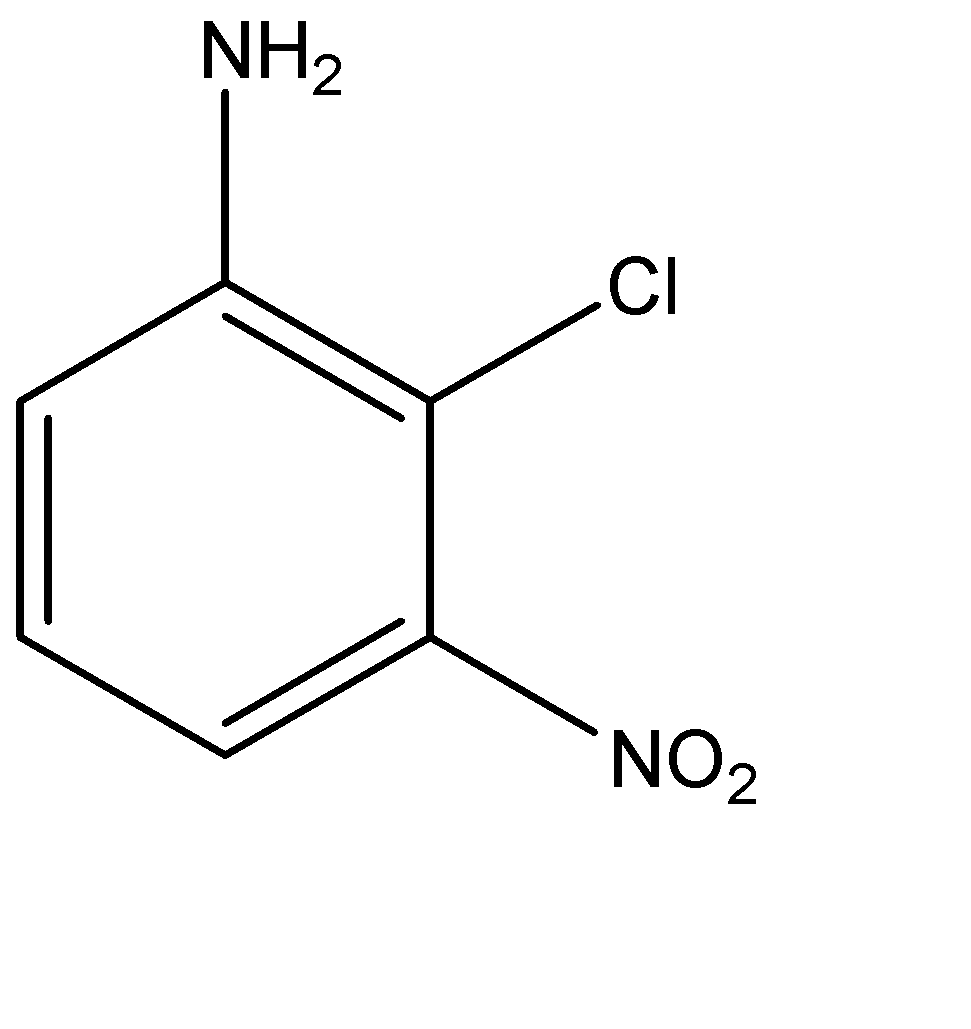
Solution
As we know that chlorination is the process of introducing a chlorine atom to the compound or molecules. Since we are given nitroaniline as a reactant to which chlorine is required to be added but while adding it we must keep in mind the various effects applied by the substituents around the benzene ring and hence answer cautiously.
Complete answer:
let us first study about the chlorination process and then further solve the reaction accordingly:-
-Chlorination: It is the process or a chemical reaction in chemistry in which chlorine atom is introduced to a molecule or a compound by the help of various methods and reagents.
-In the given reactant that is nitro aniline, we have two different substituents attached to the benzene ring which will play an important role in the addition of chlorine to it.
(A) Nitro group: It is a meta directing group as it decreases electron density at ortho and para positions with respect to itself due to which chlorine would likely get added up at meta position.
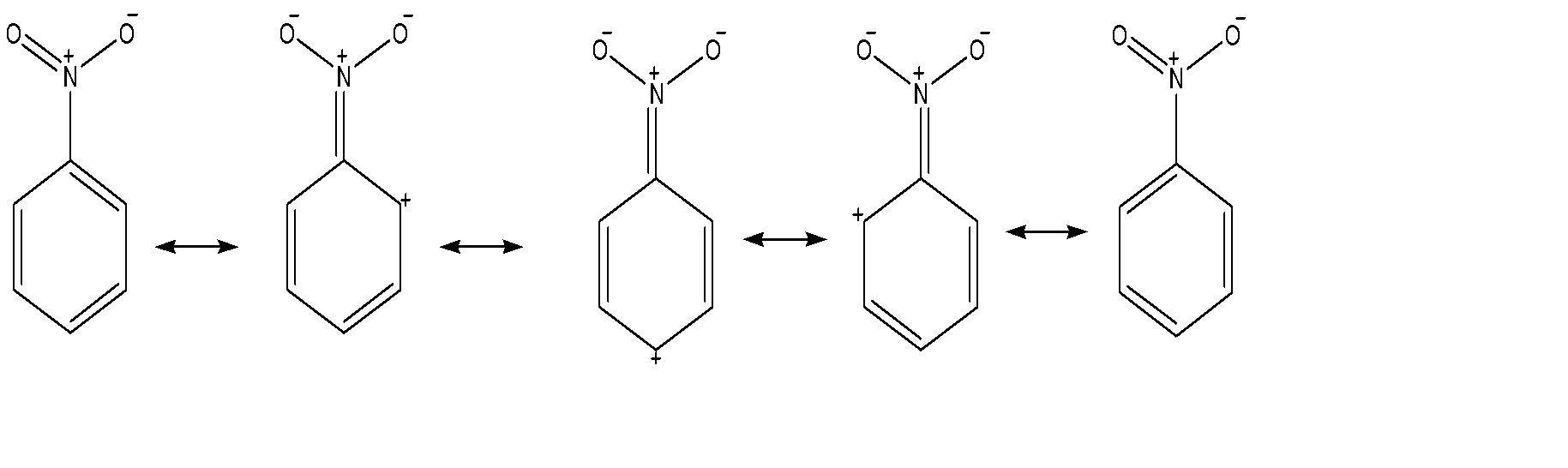
(B) Amine group: It is an ortho-para directing group as it increases electron density at ortho and para positions with respect to itself due to which chlorine would likely get added up at ortho-para position.
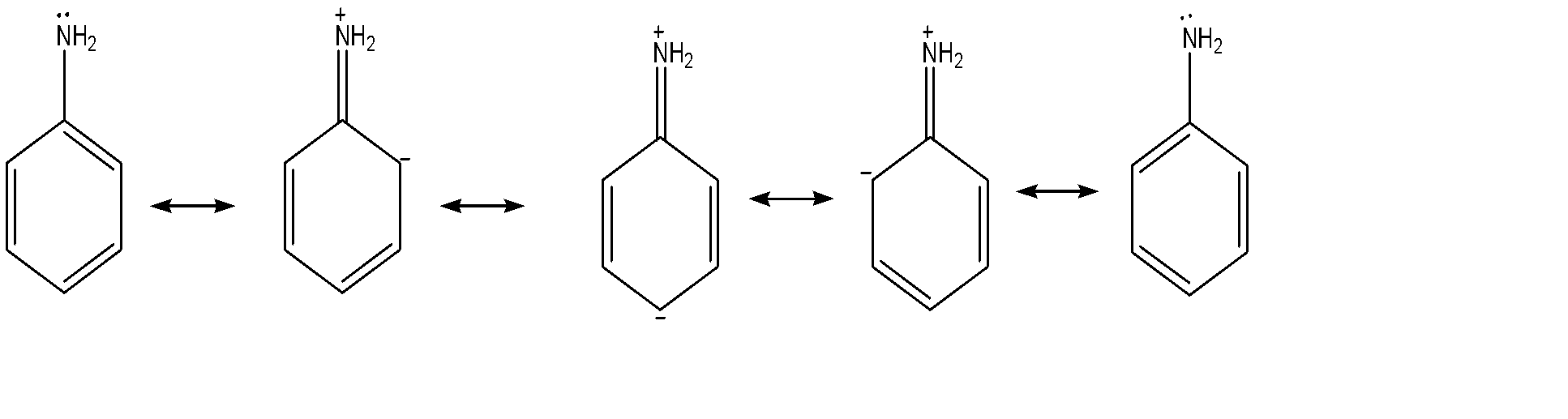
-But as we can see that there is only one charge formed on the amine group but three charges piled up on nitro group which means that amine group is more stable than nitro group, so we will perform chlorination according to chlorine and with less hindered position.
-Chlorination of nitroaniline is as follows:-
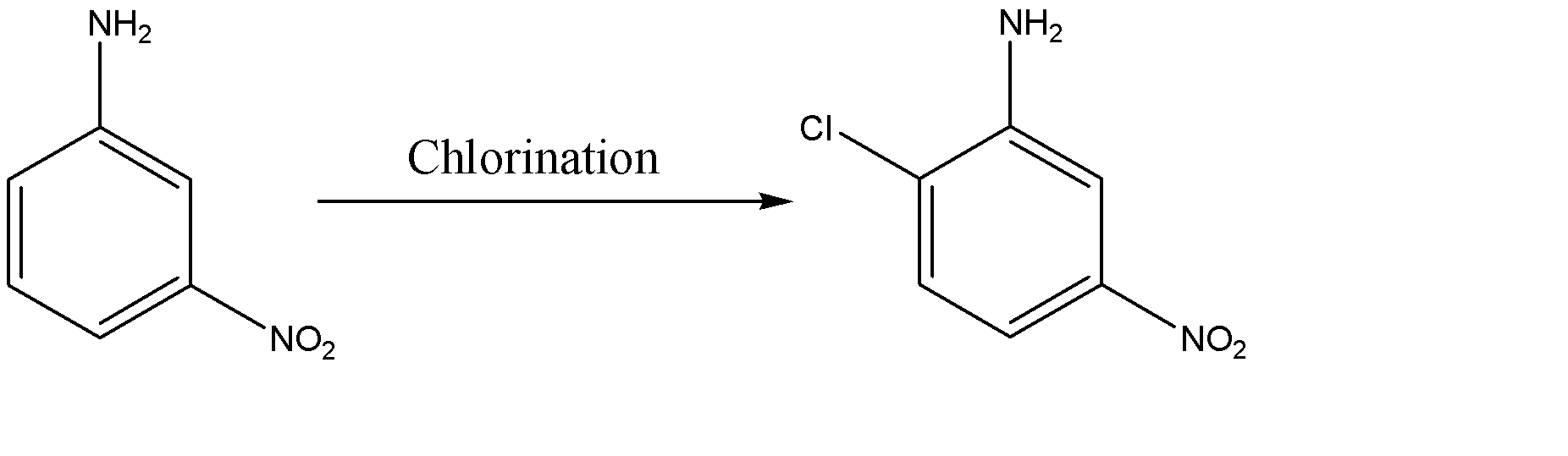
-Hence the major product formed is 2-chloro-4-nitro-aniline.
Note:
-The other ortho and para position wasn’t used for the addition of chlorine as there are steric hindrances and will form minor products.
-Always give preferences to the ring activating groups (such as −NH2,−OH,etc.) over ring deactivating groups (such as−NO2,−CHO,etc.)for desired product.
