Question
Question: If liquefied oxygen at 1 atmospheric pressure is heated from 50K to 300k by supplying heat at a cons...
If liquefied oxygen at 1 atmospheric pressure is heated from 50K to 300k by supplying heat at a constant rate. The graph of temperature vs time will be
(A)
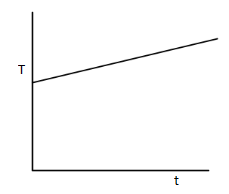
(B)
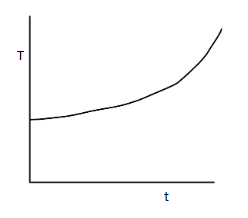
(C)
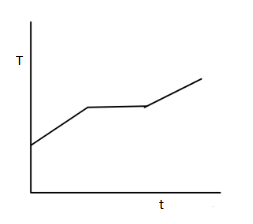
(D)
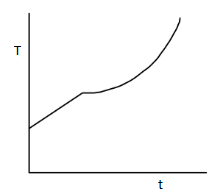
Solution
Hint : The temperature of the oxygen will rise up to its boiling point at which point the oxygen will convert to gaseous form while the temperature remains constant in the conversion. The temperature of the oxygen gas will then again begin to rise. We will use this behaviour of the oxygen gas to determine the graph of temperature vs time.
Complete step by step answer
The boiling point of oxygen is known to be 91 K. As given in the question when we start heating liquefied oxygen up from 50 K temperature the liquid oxygen will start gaining temperature up to 91 K temperature. Once it reaches 91 K temperature, the liquids oxygen will start converting into gaseous form. To convert liquid oxygen into gaseous oxygen, we must provide energy to the liquid oxygen which is also known as the latent heat of vaporization.
So, at 91 K, the temperature of the oxygen will remain stable for some time as all the liquid oxygen is converted into gaseous oxygen. Once all the oxygen is converted into gaseous form, the temperature will start rising again with time.
All of these features that were just described above correspond to the graph in option (C) which is the correct choice.
Note
In an ideal situation, the graph in option (C) is correct but in a real-world scenario, the temperature of the gas will still increase slightly when the liquid oxygen is being converted to gaseous oxygen as the energy deposition is not uniform throughout the liquid and the oxygen that has already converted to gaseous will start heating simultaneously along with the remaining liquid getting converted.
