Question
Question: If light of wavelength \(412.5nm\) is incident on each of the metals given below, which ones will sh...
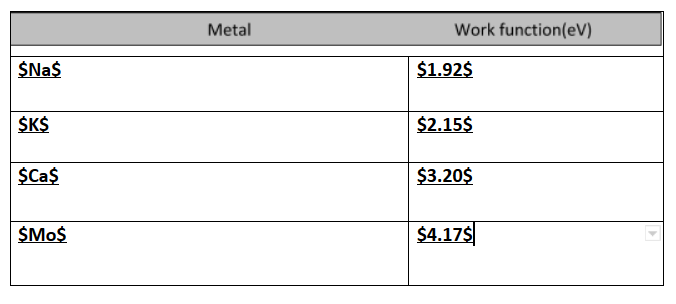
If light of wavelength 412.5nm is incident on each of the metals given below, which ones will show photoelectric emission and why?
Solution
Hint :Photoelectric effect is the emission of electrons when a electromagnetic radiation of a certain minimum frequency falls on the surface of a metal. A certain minimum frequency is required for rejecting the electron. For photoelectric emission hν=W+K.E.
Complete answer:
When light of a certain minimum frequency hits the metal surface the electrons will be just ejected. This minimum frequency required is called threshold frequency ν0. .The value hν0 is called the work function. This work function varies according to each metal surface. The light falling on the metal surface therefore should be greater than the threshold frequency.
If a electromagnetic radiation of frequency ν which is greater than the threshold frequency falls on the metal surface the additional energy will be given to the electron as kinetic energy. This can be written in equation form as hν=hν0+KE. Here KE represents the kinetic energy.
Here given that light falling gas a wavelength of 412.5nm .Therefore using equation ν=λc we get the frequency of the incident light is
=412.5×10−93×108
=7.27×1014
Calculating its energy and converting it into electron volts (1J=6.242×1018eV)
E=hν=6.626×10−34× 7.27×1014×6.6242×1018
=3eV
Therefore all the metals having a work function less than 3eV will be ejected.Na and K which has a work function of 1.92 and 2.15 will eject electrons.
Note When the wavelength of the incident light is given its energy in electron volts is directly given by the equation (by substituting all other constant values)E=λ(innm)1240eV
Substituting the wavelength here we get the energy in electron volt is equal to 3eV.
Memorising this equation will help to solve the question faster.
