Question
Question: If Hund’s rule is not followed, magnetic moment of \(F{e^{2 + }},M{n^ + }{\text{ and Cr}}\) all havi...
If Hund’s rule is not followed, magnetic moment of Fe2+,Mn+ and Cr all having 24 electrons will be in order:
(A) Fe2+,Mn+<Cr
(B) Fe2+=Cr<Mn+
(C) Fe2+=Mn+<Cr
(D) Mn+=Cr<Fe2+
Solution
Hund’s rule states that electrons arrange in the orbitals in a way that the spin of the electrons remains maximum. The atomic number of Fe, Mn and Cr is 26, 25 and 24 respectively.
Complete Step-by-Step Solution:
We need to compare the magnetic moment of three species given to us. It is given to suppose that Hund’s rule is not followed when the electrons are arranged in the orbitals.
- Hund’s rule states that electrons arrange in the orbitals in a way that the spin of the electrons remains maximum. So, it is also called maximum spin multiplicity rule. Now, we will write the new electronic configuration of given species if Hund’s rule is not followed.
- Electronic configuration of Fe2+: [Ar]3d6
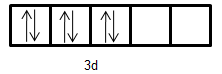
- Electronic configuration of Mn+: [Ar]3d54s1
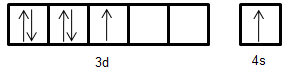
Electronic configuration of Cr: [Ar]3d44s2
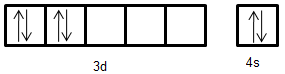
- Thus, from the above given electronic configurations we can say that Fe2+ does not have any unpaired electron. Mn+ has two unpaired electrons. Cr atoms also do not have any unpaired electrons.
- We know that the magnetic moment arises from the spin of unpaired electrons.
- So, we can say that the magnetic moment of both Fe2+ and Cr atoms will be zero. Thus, the magnetic moment of both of them is the same.
- The magnetic moment of Mn+ will be some positive value as it has two unpaired electrons.
- Thus, we can say that the order of magnetic moment of these ions will be Fe2+=Cr<Mn+
So, the correct answer to this question is (B).
Note: Do not forget to arrange the electrons in a way the spin of the electrons remains as less as possible here as Hund’s rule is not followed. Actually, Hund’s rule is followed in nature and Fe2+ ion has 4, Mn+ has 6 and Cr has six unpaired electrons.
