Question
Question: If E= the number of lone pairs of electrons on\[Xe\], B = the number of bonding pairs of electrons...
If E= the number of lone pairs of electrons onXe,
B = the number of bonding pairs of electrons,
S = shape of the molecule,
Then what is the correct set of E, B and S of XeF4?
a.) E-3, B-3, S- octahedral
b.) E-3, B-3, S-square planer
c.) E-2, B-4, S- square planer
d.) E-4, B-2, S-square planer
Solution
For answering this question we should collect all information about Xe. It is a last group element with 8 valence electrons means it has 8 electrons in its outermost shell. And to check the number of bonding pairs we will see how many bonds it is making with F and for shape we will check which positions of F make the molecule more stable.
Complete step by step answer:
We know that Xe has 8 numbers of valence electrons and 4 lone pairs. InXeF4 , Xe shares 4 numbers of electrons with 4 fluorines to complete its octet. We know that valency of fluorine is one and it requires one electron to complete its octet.
So, Xe has 4 electrons left or 2 lone pairs with it. Here the number of lone pairs of electrons at Xe in XeF4 is 2. Which means E = 2.
And here the number of bond pairs (bond with fluorine), B = 4.
And for the shape of XeF4:
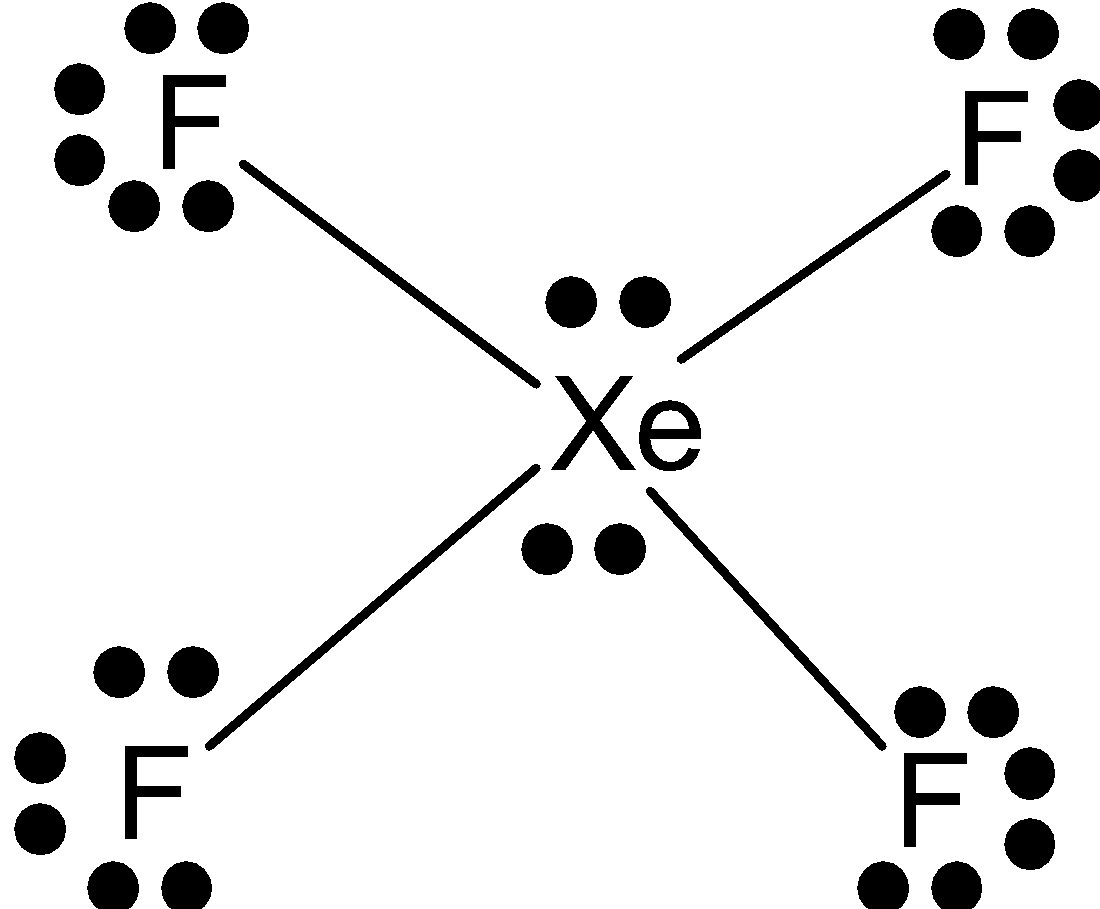
So, the structure or shape of XeF4 is square planar. All four fluorines are in the same plane and one lone pair is above the plane and one is below the plane.
S = square planer
E =2
B = 4
So, the correct answer is “Option C”.
Note: Here the hybridization of Xe is sp3d2. In which two p-orbitals and 2 d-orbitals are making bonds with fluorine and rest two are lone pairs. From the hybridization, we assume that the shape should be octahedral but because of lone pairs it is square planar.
