Question
Question: If $\Delta G^\circ$ of acidic solution of a half cell $MnO_4^-/MnO_2$ is -xF. Calculate value of x. ...
If ΔG∘ of acidic solution of a half cell MnO4−/MnO2 is -xF. Calculate value of x.
(EMnO4−/Mn2+∘=1.5V,EMnO2/Mn2+∘=1.25V)
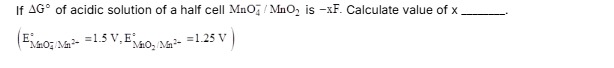
5
Solution
To calculate the value of x, we need to determine the standard Gibbs free energy change (ΔG∘) for the half-cell reaction MnO4−/MnO2 in acidic solution. The reaction can be written as: MnO4−→MnO2
First, let's write the given half-reactions and their corresponding ΔG∘ values. The relationship between ΔG∘ and standard electrode potential (E∘) is given by: ΔG∘=−nFE∘ where n is the number of electrons transferred, and F is Faraday's constant.
1. For EMnO4−/Mn2+∘=1.5V: The half-reaction is MnO4−→Mn2+. The oxidation state of Mn in MnO4− is +7. The oxidation state of Mn in Mn2+ is +2. The change in oxidation state is 7−2=5. So, 5 electrons are involved. The balanced half-reaction in acidic medium is: MnO4−+8H++5e−→Mn2++4H2O (Reaction 1) Here, n1=5 and E1∘=1.5V. ΔG1∘=−n1FE1∘=−5×F×1.5=−7.5F
2. For EMnO2/Mn2+∘=1.25V: The half-reaction is MnO2→Mn2+. The oxidation state of Mn in MnO2 is +4. The oxidation state of Mn in Mn2+ is +2. The change in oxidation state is 4−2=2. So, 2 electrons are involved. The balanced half-reaction in acidic medium is: MnO2+4H++2e−→Mn2++2H2O (Reaction 2) Here, n2=2 and E2∘=1.25V. ΔG2∘=−n2FE2∘=−2×F×1.25=−2.5F
3. For the target half-cell MnO4−/MnO2: The half-reaction is MnO4−→MnO2. The oxidation state of Mn in MnO4− is +7. The oxidation state of Mn in MnO2 is +4. The change in oxidation state is 7−4=3. So, 3 electrons are involved. The balanced half-reaction in acidic medium is: MnO4−+4H++3e−→MnO2+2H2O (Reaction 3) Let this be ΔG3∘.
We can obtain Reaction 3 by combining Reaction 1 and Reaction 2. We want MnO4− on the reactant side and MnO2 on the product side. Reaction 1: MnO4−+8H++5e−→Mn2++4H2O To get MnO2 on the product side, we need to reverse Reaction 2: Mn2++2H2O→MnO2+4H++2e− (Reversed Reaction 2) The ΔG∘ for the reversed reaction is the negative of the original ΔG∘: ΔG2,reversed∘=−ΔG2∘=−(−2.5F)=2.5F
Now, add Reaction 1 and Reversed Reaction 2: (MnO4−+8H++5e−→Mn2++4H2O) + (Mn2++2H2O→MnO2+4H++2e−)
MnO4−+(8−4)H++(5−2)e−+(2−4)H2O→MnO2 MnO4−+4H++3e−→MnO2+2H2O This is exactly Reaction 3.
Since Gibbs free energy is an additive property, ΔG3∘ can be calculated as: ΔG3∘=ΔG1∘+ΔG2,reversed∘ ΔG3∘=−7.5F+2.5F ΔG3∘=−5.0F
The problem states that ΔG∘ for this half-cell is -xF. Comparing −xF with −5.0F: −xF=−5.0F x=5.0
The final answer is 5.
Explanation: The standard Gibbs free energy change (ΔG∘) is an additive property. We are given standard electrode potentials (E∘) for two half-reactions: MnO4−/Mn2+ and MnO2/Mn2+. We need to find ΔG∘ for the MnO4−/MnO2 half-reaction. We convert each E∘ into ΔG∘ using ΔG∘=−nFE∘. Then, we manipulate the given half-reactions to obtain the desired half-reaction and sum their corresponding ΔG∘ values.
- For MnO4−+8H++5e−→Mn2++4H2O, ΔG1∘=−5F(1.5V)=−7.5F.
- For MnO2+4H++2e−→Mn2++2H2O, ΔG2∘=−2F(1.25V)=−2.5F. To get MnO4−→MnO2, we subtract the second reaction from the first (or add the reverse of the second reaction). The target reaction is MnO4−+4H++3e−→MnO2+2H2O. ΔGtarget∘=ΔG1∘−ΔG2∘=−7.5F−(−2.5F)=−7.5F+2.5F=−5.0F. Given ΔG∘=−xF, therefore x=5.0.
