Question
Question: IF \( Ca{C_2}\xrightarrow{{Hydrolysis}}A\xrightarrow{{HgS{O_4} + {H_2}S{O_4}}}B \) Then B is : ...
IF CaC2HydrolysisAHgSO4+H2SO4B
Then B is :
(A) Acetylene
(B) Acetaldehyde
(C) Acetone
(D) Acetic acid
Solution
Hint : Calcium carbide is a compound with carbon and calcium. It undergoes hydrolysis to form acetylene. Further, when acetylene treated with mercuric sulphate and sulphuric acid the acetylene forms enol compounds. This enol compound undergoes tautomerism to form aldehyde known as acetaldehyde.
Complete Step By Step Answer:
Calcium carbide is a chemical compound with the molecular formula of CaC2 .it contains a calcium ion and two carbon atoms. It is mainly used for the ripening of fruits such as mangoes. But it is not good for your health. Thus, nowadays it was banned.
Hydrolysis is the chemical process of addition of water molecules. Calcium carbide on hydrolysis forms acetylene. Acetylene is an alkyne. It contains two carbon atoms. The molecular formula of acetylene is C2H2 . The structure of acetylene is CH≡CH . Thus, it has a triple bond and is referred to as an unsaturated compound.
Acetylene upon treatment with mercuric sulphate and sulphuric acid forms an enol compound. The enol compound undergoes tautomerism to form a corresponding aldehyde.
Thus, acetylene forms acetaldehyde. Acetaldehyde is a compound with the molecular formula of CH3CHO . The functional group present is carbonyl group, ketones and aldehyde belong to carbonyl groups.
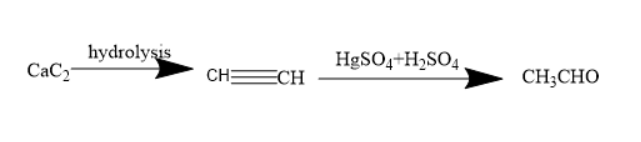
Thus, the compound B is acetaldehyde.
Option B is the correct one.
Note :
The enol compound has a double bond and hydroxyl group. It undergoes tautomerization. The transfer of one hydrogen atom within the compound forms a carbonyl compound aldehyde or ketone. The enol compound forms at first, undergoes tautomerization to form acetaldehyde.
