Question
Question: If at 200 K & 500 atm, density of $CH_4$ is 0.25 gm/ml then its compressibility factor (Z) is...
If at 200 K & 500 atm, density of CH4 is 0.25 gm/ml then its compressibility factor (Z) is
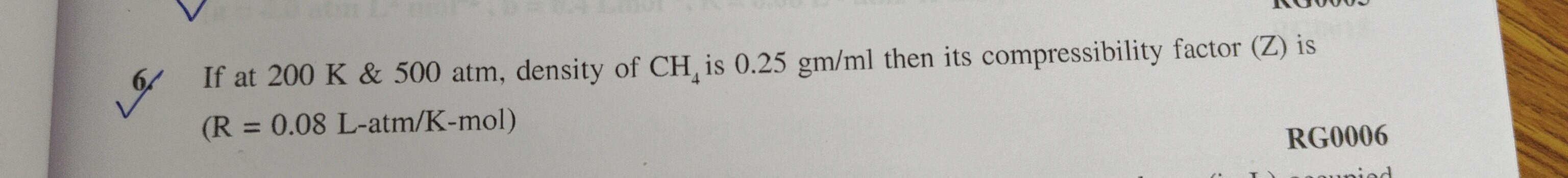
A
1
B
2
C
0.5
D
0.25
Answer
2
Explanation
Solution
The compressibility factor (Z) is calculated using the formula Z=ρRTPM. First, convert the density from g/ml to g/L: 0.25 g/ml=250 g/L. The molar mass of CH4 is 16 g/mol. Substitute the given values: P = 500 atm, M = 16 g/mol, ρ = 250 g/L, R = 0.08 L-atm/K-mol, and T = 200 K. Z=(250 g/L)×(0.08 L-atm/K-mol)×(200 K)(500 atm)×(16 g/mol)=40008000=2.
