Question
Question: If a metal has a bcc structure, then why is its coordination number is 8? A.Each atom touches four...
If a metal has a bcc structure, then why is its coordination number is 8?
A.Each atom touches four atoms in the layer above it, four in the layer below it and none in its own layer.
B.Ten atom touches four atoms in the layer above it, four in the layer below it and one in its own layer.
C.One atom touches four atoms in the layer above it, four in the layer below them and none of their own layer.
D.Each atom touches eight atoms in the layer above it, eight in the layer below it and none in its own layer.
Solution
In a crystal structure system, the point sets and the corresponding space sets define the lattice system. Coordination number is determined by the share of nearest neighbouring atoms or ions covering the atom or ion.
Complete answer:
To understand and simplify this question, first we need to go through the lattice system.
Basically, there are 14 ‘Bravais lattice structures’, and these are grouped into 7main categories or crystal lattices. These 7 groups are – cubic, rhombohedral, triclinic, orthorhombic, monoclinic, tetragonal, and hexagonal.
A BCC or Body centered cubic structure is a unit cell having a total of 2 atoms – eight-eighths from the corner and one at the centre. As we know that atom at the corner of the cell is surrounded by eight neighbouring atoms, so its share to the unit cell is \defrac18 . And since there are 8 corners in the unit cell, this gives us a complete 1 atom.
Like, 8(corners)×81+1(at the centre of the cell) = 2 atoms
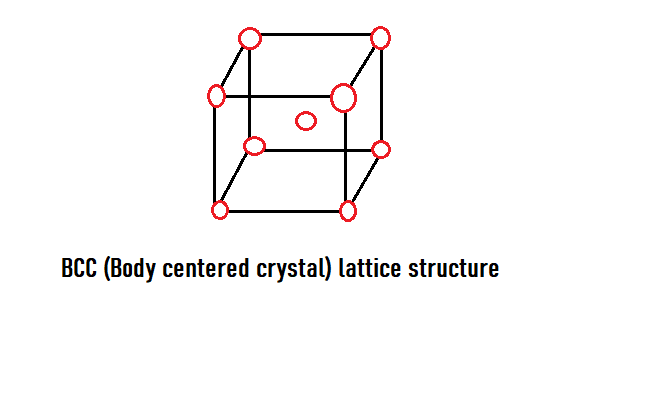
So, now we understand the structure of the bcc unit cell, this will help us find the coordination number easily.
Now we know that the central atom of the bcc unit cell is touched by the atoms of the 8 corners.
Thus, the coordination number of the bcc crystal structure is8.
Note :
When we talk about the packing efficiency of the unit cell, it is 68%in the case of body centered cubic structure. So packing efficiency is the ratio of the space occupied by the spherical atoms to the total volume of the solid.
