Question
Question: If a graph is plotted with the atomic number on x-axis and the square root of the frequency of a spe...
If a graph is plotted with the atomic number on x-axis and the square root of the frequency of a spectral line of characteristic X-rays on the y-axis, then it will be
A) A straight line passing through the origin
B) A parabola
C) A straight line not passing through the origin
D) A rectangular hyperbola
Solution
The atomic number or proton number is the number of protons found in the nucleus. A spectral line is a characteristic line in the entire continuous spectrum of an element, resulting from light emission and absorption in a narrow range of frequency when compared with the nearby frequencies.
Complete step by step solution:
The atomic number of an element can be related to the square root of the frequency of a spectral line of characteristic X-rays using Moseley’s law. Moseley’s law is an empirical law concerning the characteristic X-rays emitted by atoms. It states that the square root of the frequency of emitted X-rays is approximately proportional to the atomic number.
Mathematically, the law can be represented using the equation υ∝Z where υ is the frequency of the emitted X-ray.
The final equation of Moseley’s law can be written as υ=A(Z−b)2 where and are constants depending upon the X-ray emission. Moseley measured and plotted the X-ray frequencies of about 40 elements of the periodic table following his law and observed the graph to be a straight line. The plot of the graph with atomic numbers on the x-axis and the square root of frequencies on the y-axis furnished a straight line not passing through the origin.
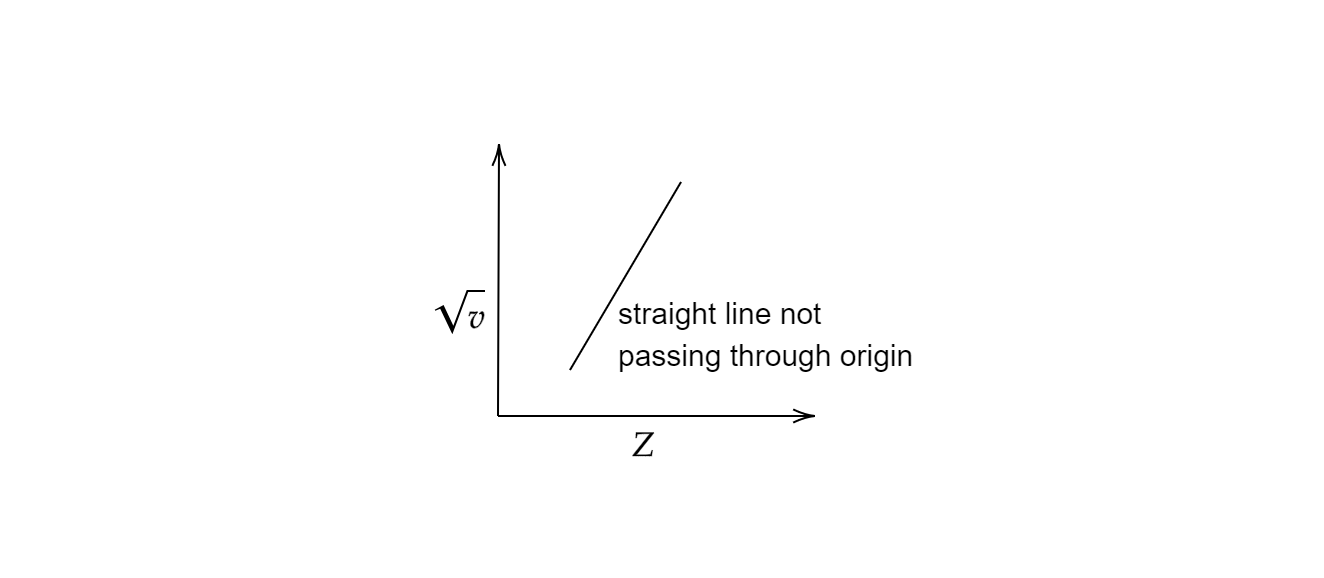
Hence, option (C) is the correct option.
Note: Moseley’s law was the first law to associate the atomic number of elements with any known physical quantity. It established the atomic number as a measurable experimental quantity and also gave it a viable physical meaning. Henry Moseley, the physicist behind this law, has also made major contributions to the periodic table.
