Question
Question: If 1.26g of oxalic acid is dissolved in 250 ml of solution, find its normality. The equivalent mass ...
If 1.26g of oxalic acid is dissolved in 250 ml of solution, find its normality. The equivalent mass of oxalic acid is 63.
Solution
Normality is defined as the number equivalents of solute dissolved per litre of solution. Normality is the expressions used to measure the concentration of a solution. It is abbreviated as ‘N’ and is sometimes referred to as the equivalent concentration of a solution.
Step by step solution:
- Molecular formula of oxalic acids in dehydrate form is:
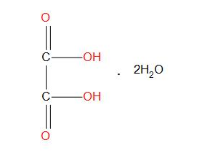
- Weight of sample taken is =1.26g
Equivalent weight is given that is= 63
-The volume of solution =250 ml
- Now, let’s calculate the normality of the solution,
Formula for calculating normality is:
Normality =equivalent weight of sampleweight of sample taken×volume of solution1000
So, by solving we get,
Normality=631.26×2501000
=0.08N
Hence, we can say that the normality of the solution is 0.08 N.
Additional information:
- It is found that Many chemists use normality in acid-base chemistry to avoid the mole ratios in the calculations or we can say simply to get more accurate results. while normality is used commonly in precipitation and redox reactions. There are some Limitations found in using Normality, some of them are:
- It is not a proper unit of concentration. It is an ambiguous measure and we can say that molarity or molality are better options for units.
- It is also found that normality is not a specified value for a particular chemical solution. The value can significantly change depending on the chemical reaction. To elucidate further, we can say that one solution can actually contain different normalities for different reactions.
Note: We should not get confused in terms of normality and molarity. Normality is the number equivalent of solute dissolved per litre of solution. And, Molarity is defined as the number of moles of solute per litre of solution. The unit of normality is N. The unit of molarity is M.
