Question
Question: Identify \(Z\) in the sequence.  A. C6H5CN
A. C6H5CN
B. C6H5CONH2
C. C6H5COOH
D. C6H5CH2NH2
Solution
Hint: Aniline is an oily , colourless compound . In amine it is made up of an amino group attached to a benzene ring. It can oxidize slowly. It has the smell of unpleasant rotten fish and has a burning aromatic taste. It reacts with strong acid and forms salts like anilinium, acetyl chloride , acetanilide. Aniline derivatives are highly reactive in electrophilic substitution. It does not evaporate at room temperature. Aniline is used to make dyes, rubber additives, drugs, polymer ,pesticides and in the manufacture of other important chemicals. When aniline is exposed to air it is broken down by sunlight.
Complete answer:
When aniline is treated with NaNO2+HCl it will produce benzene diazonium chloride C6H5N2Cl,This reaction is done at 273K so that further reduction can be prevented. In benzene diazonium chloride nitrogen and nitrogen have a triple bond between them. So in the given reaction X is benzene diazonium chloride salt. This reaction is also called diazotization.
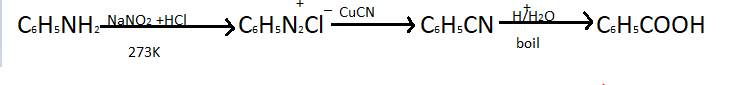 When benzene diazonium chloride further reacts with CuCNit forms benzonitrile which is a member of benzene and cyanide.This is a colourless aromatic compound. It is also known as cyano benzene or phenyl cyanide.In the given sequence Y is cyanobenzene . When benzonitrile/cyano benzene reacts with water it gives C2H5COOH. So Z is ethanoic acid in the given reaction.
When benzene diazonium chloride further reacts with CuCNit forms benzonitrile which is a member of benzene and cyanide.This is a colourless aromatic compound. It is also known as cyano benzene or phenyl cyanide.In the given sequence Y is cyanobenzene . When benzonitrile/cyano benzene reacts with water it gives C2H5COOH. So Z is ethanoic acid in the given reaction.
Note: So in this problem we are following the approach of getting the final product of the given reactions we need to solve one by one reaction .As the whole reaction is the process of preparation of ethanoic acid from aniline.So option C2H5COOH is the correct answer.
