Question
Question: Identify 'Z' in the following series of reaction: $Butan-2-ol \xrightarrow{PCl_5} X \xrightarrow{al...
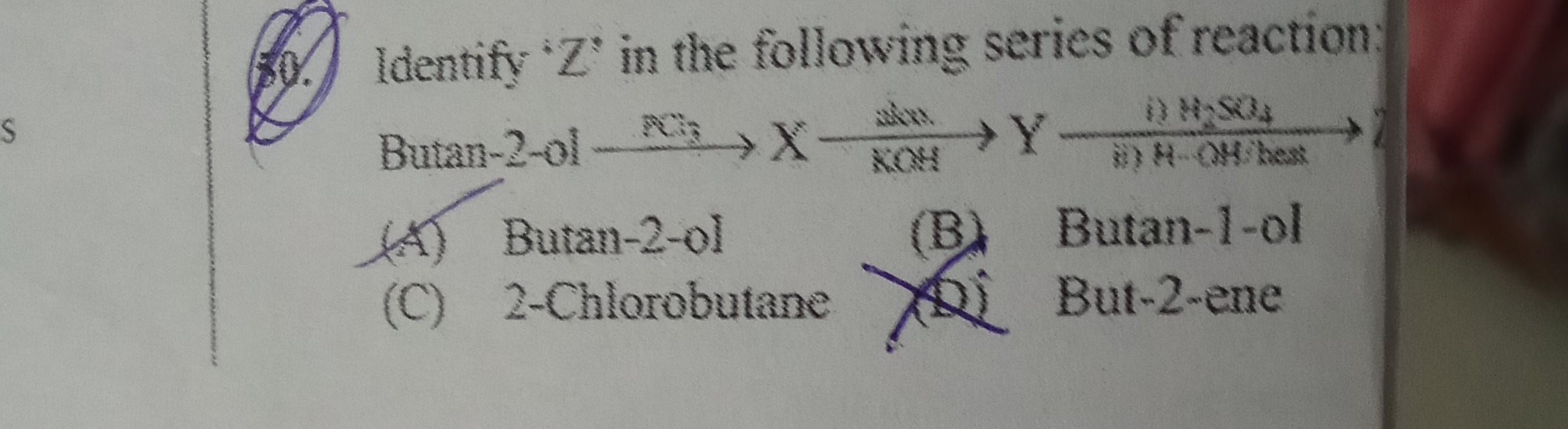
Identify 'Z' in the following series of reaction:
Butan−2−olPCl5Xalc.Yⅰ)H2SO4ii)H−OH/heatZ
A
Butan-2-ol
B
Butan-1-ol
C
2-Chlorobutane
D
But-2-ene
Answer
Butan-2-ol
Explanation
Solution
-
Step 1:
Butan-2-olPCl52‑Chlorobutane (X)
Butan-2-ol reacts with PCl₅ to form 2‑chlorobutane. -
Step 2:
Under alcoholic conditions, 2‑chlorobutane undergoes dehydrohalogenation (elimination) following Zaitsev’s rule to give the more substituted alkene, but‑2‑ene (Y). -
Step 3:
But‑2‑eneH2SO4,H2O/heatButan-2-ol (Z)
But‑2‑ene, when treated with H₂SO₄ (acid-catalyzed hydration) followed by H₂O/heat, undergoes Markovnikov addition of water to regenerate butan‑2‑ol (Z).
Explanation (minimal):
Butan-2-ol → 2‑chlorobutane (with PCl₅) → but‑2‑ene (elimination under alcoholic conditions) → butan‑2‑ol (hydration with H₂SO₄/H₂O, heat) due to Markovnikov addition.
