Question
Question: Identify ‘Z’ in the following reaction series: \(C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Br\xrightarrow{NaOH...
Identify ‘Z’ in the following reaction series:
CH3CH2CH2BrNaOH(X)Al2O3Δ(Y)HOCl(Z)
(A) 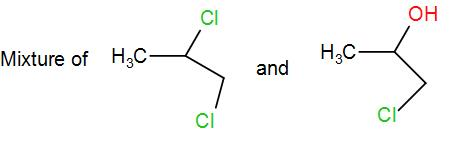
(B) 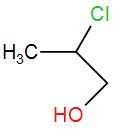
(C) 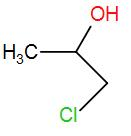
(D) 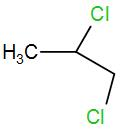
Solution
Here, a bromoalkene is given to you that will undergo a series of reactions upon addition of the given reagents. To find the ‘Z’ in the reaction, precede stepwise by finding X and Y and then arriving at Z. Remember that base will form alcohol from the bromo derivative. The aluminium oxide will form an alkene from the alcohol and HOCl will chlorinate the alkene. Use this to answer the given question.
Complete step by step solution:
In the given question, a bromo derivative of an alkane is given to us. Its name is 1-bromopropane or is also known as n-propyl bromide.
To find ‘Z’ in the given reaction series, let us see the effects of the added reagents and then arrive at the final result.
Firstly, a base is added to the bromo derivative. The base will reduce the derivation to give us alcohol. This will give us propanol. We can write the reaction as-
CH3CH2CH2BrNaOHCH3CH2CH2OH
Thus the alcohol formed is our ‘X’. To this aluminium oxide is added and the solution is heated. Aluminium oxide forms terminal alkenes from alcohol. We can write the reaction as-
CH3CH2CH2OHAl2O3ΔCH3−CH=CH2
The alkene thus obtained is our ‘Y’. To this, HOCl is added. This will be a chlorination reaction that is the alkene will be chlorinated. The terminal alkene will be halogenated and will give rise to a carbocation. We can write the reaction as-
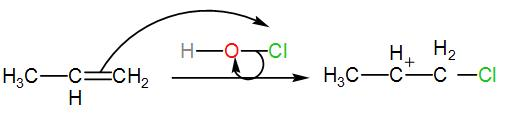
The carbocation thus formed will react with the free hydroxide ion which is eliminated from HOCl when the alkene is chlorinated. This will give us chlorinated alcohol. We can write the reaction as-
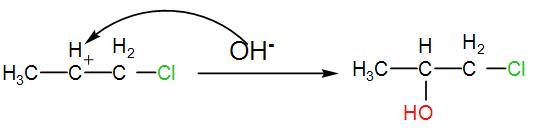
The product obtained is 4-chloropropan-2-ol. This is the required ‘Z’.
Therefore, the correct answer is option (C).
Note: Alkene halogenation is generally carried out for bromination and chlorination. This is done using dihalides like Cl2 and Br2. After breaking the double bond the di-halide is added and here the halide gets attached to the neighbouring carbons from the opposite faces of the molecules.
