Question
Question: Identify \[\;x,y\] and \[z\]. 
Solution
The nitro benzene chloride is the organic compound which a yellow crystalline solids. It is as important as the precursor to the other compounds because it consists of two functional groups. It has the tendency to dissolve in diethyl ether, benzene and hot ethanol. It does not get soluble in water.
Complete step by step answer:
So when 2- nitrobenzene chloride reacts with sodium hydroxide at the temperature of 443K or 170oC it forms 2- nitrophenol. So here the xoC in the reaction or the equation is equal to 170oC. The equation for the reaction of 2- nitro benzene chloride is the following:
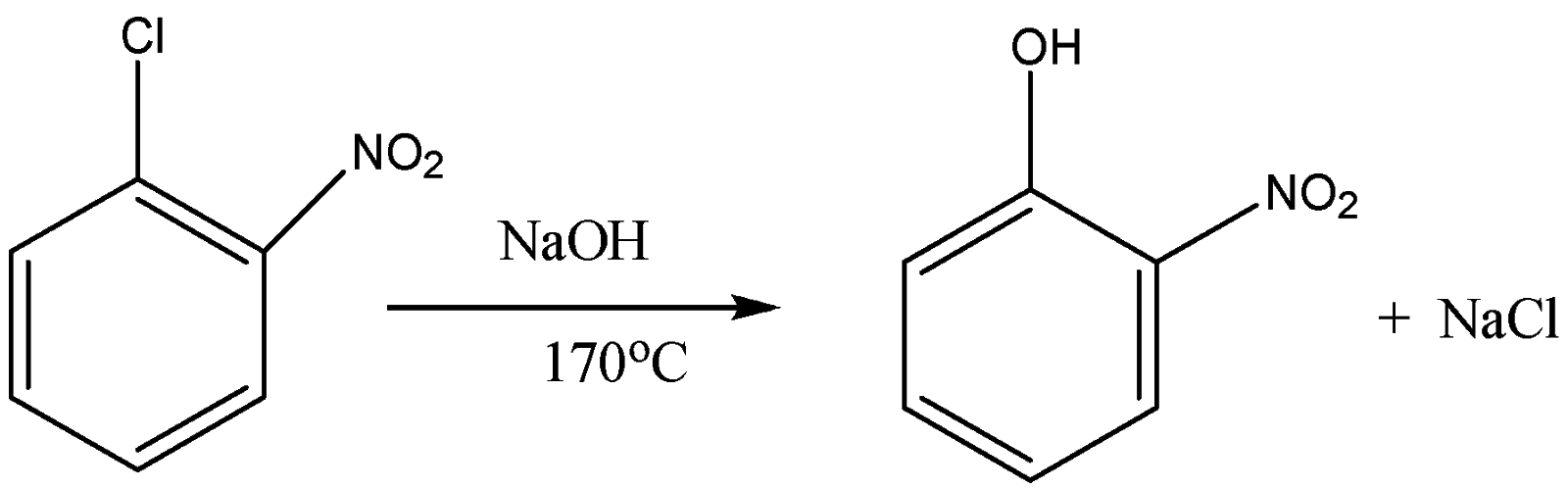
So the product formed is 2- nitrophenol and the answer for xoC is 170oC.
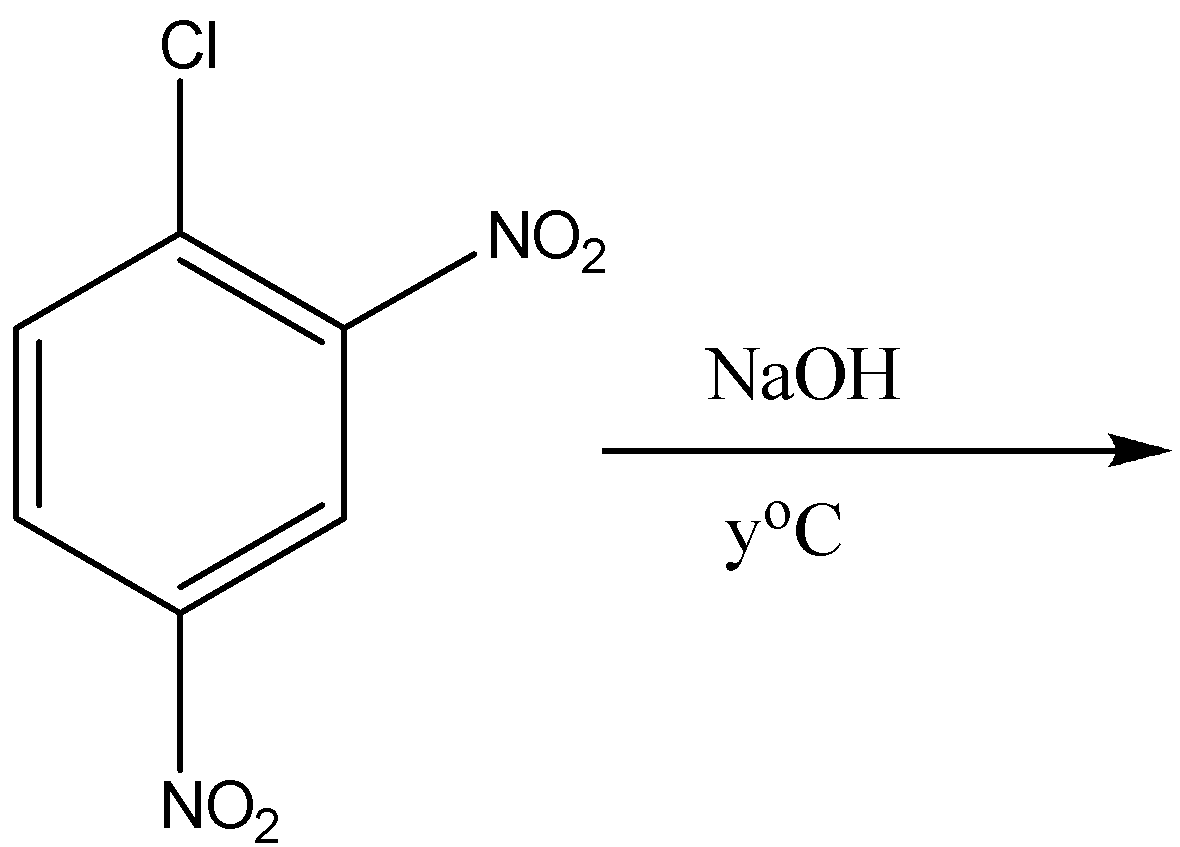
Now on coming to second part that is 2,4 dinitro benzene chloride, when it reacts with the sodium hydroxide at the temperature of 100oC or 373K the formation of 2,4 dinitro phenol takes place. So the answer of yoC in this reaction is 100oC. Now the equation for the 2,4-dinitro benzene chloride is the following:
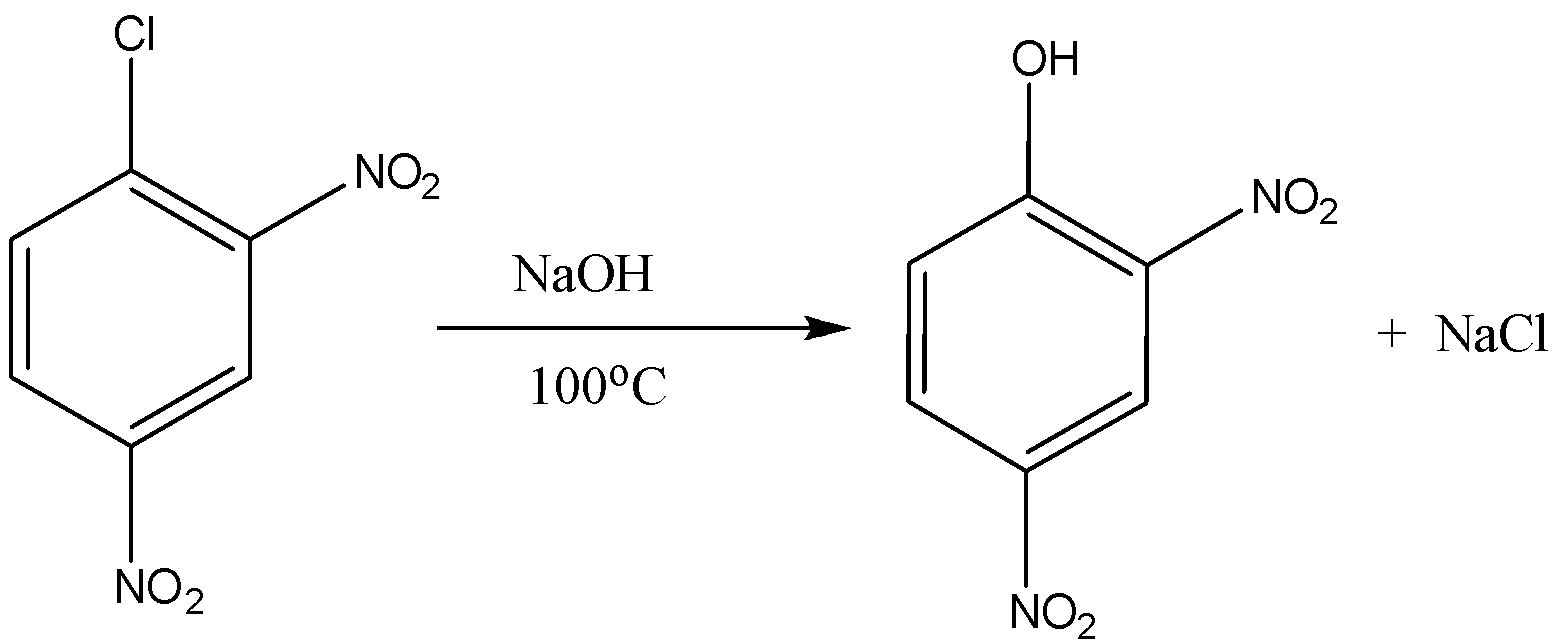
So the product formed is 2,4 dinitro phenol and the answer of yoC is 100oC.
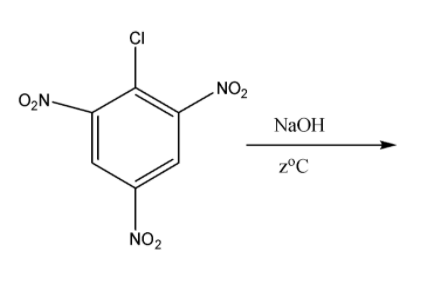
Now we come to the third part in which we have 2,4,6-trinitro benzene chloride. When it is reacted with sodium hydroxide with warm water at the temperature of 333K or 60oC the formation of 2,4,6-tinitro phenol or picric acid takes place. The reaction for them is the following:
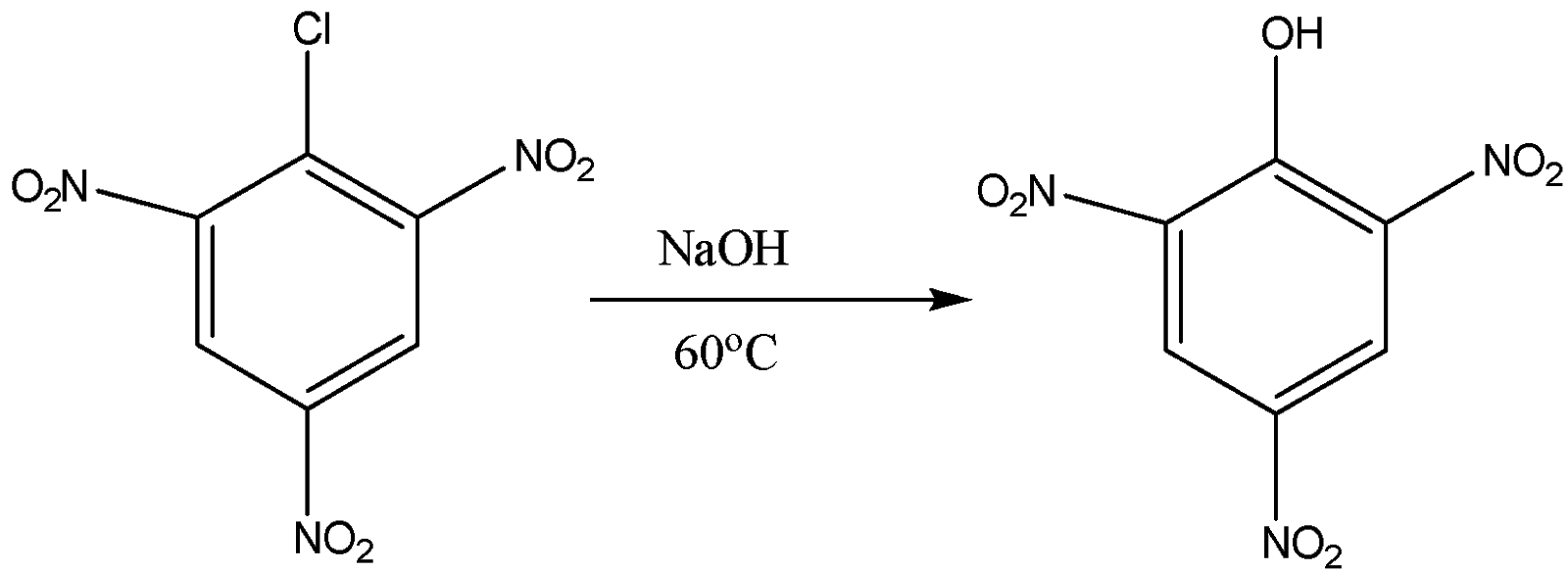
So the product formed is picric acid and the answer for zoC is 60oC.
Note: picric acid is toxic in nature and has the pH of 1.3. It is a strong acid and highly corrosive in nature as well as irritant to eyes too.it is pale yellow in colour which is a crystalline solid used in military explosives. It is used as a yellow dye and antiseptic. It is disposed of by incineration because it is dangerous.
