Question
Question: Identify X in the series, $\xrightarrow[\text{H}_2\text{SO}_4]{\text{HNO}_3}$ Intermediate $\xright...
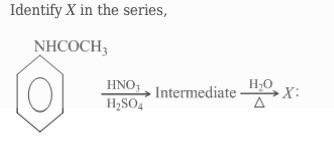
Identify X in the series,
HNO3H2SO4 Intermediate H2OΔX:
X is a mixture of ortho-nitroaniline and para-nitroaniline, with para-nitroaniline being the major product due to steric hindrance.
Solution
The reaction starts with acetanilide, which undergoes nitration using HNO3/H2SO4. The −NHCOCH3 group is an activating group and an ortho, para director, leading to nitration at both positions. Para-nitroacetanilide is the major product due to steric hindrance.
Hydrolysis of the intermediate (a mixture of ortho-nitroacetanilide and para-nitroacetanilide) with water and heat cleaves the amide bond, forming an amine and a carboxylic acid. This yields ortho-nitroaniline and acetic acid from ortho-nitroacetanilide, and para-nitroaniline and acetic acid from para-nitroacetanilide.
Therefore, X is a mixture of ortho-nitroaniline and para-nitroaniline, with para-nitroaniline as the major product.
