Question
Question: Identify undergoing oxidation species in following reaction. $A + B \longrightarrow A^+ + B^{2-}$...
Identify undergoing oxidation species in following reaction.
A+B⟶A++B2−
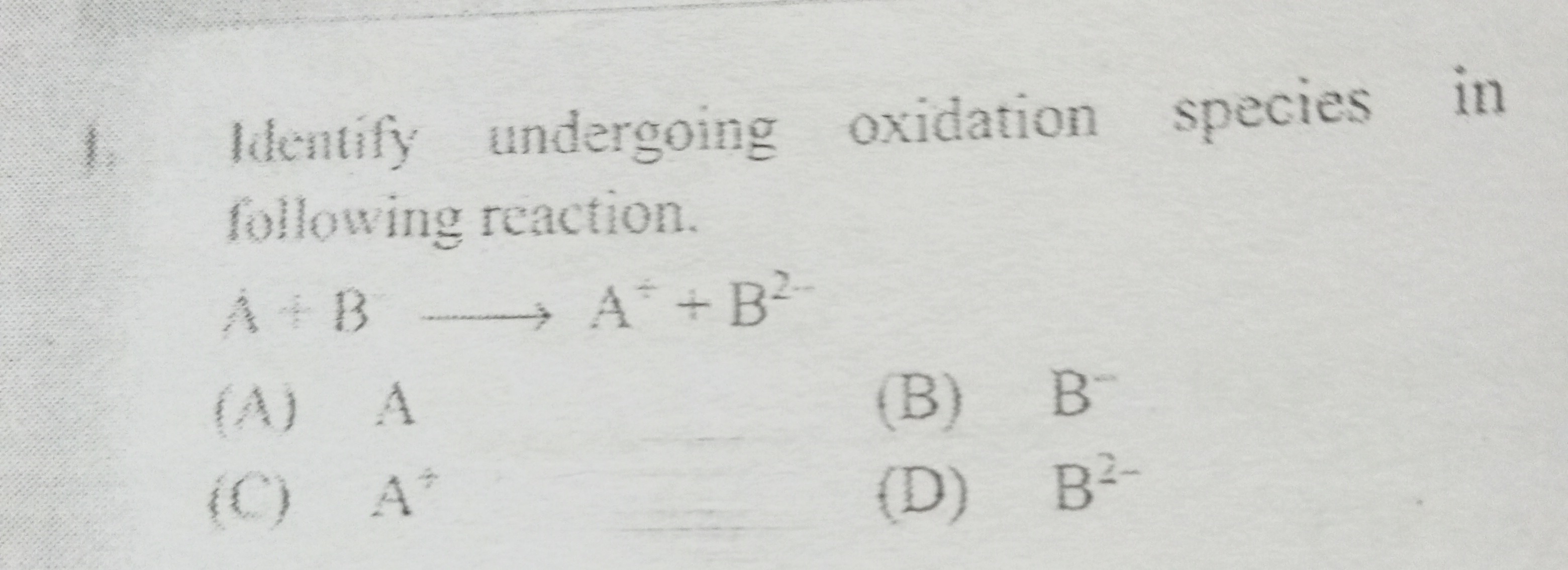
A
A
B
B−
C
A+
D
B2−
Answer
A
Explanation
Solution
In the reaction, A is converted to A⁺, which implies that A loses an electron. Loss of electrons is oxidation. Therefore, A is undergoing oxidation.
