Question
Question: Identify undergoing oxidation species in following reaction. $A + B \longrightarrow A^+ + B^{2-}$...
Identify undergoing oxidation species in following reaction.
A+B⟶A++B2−
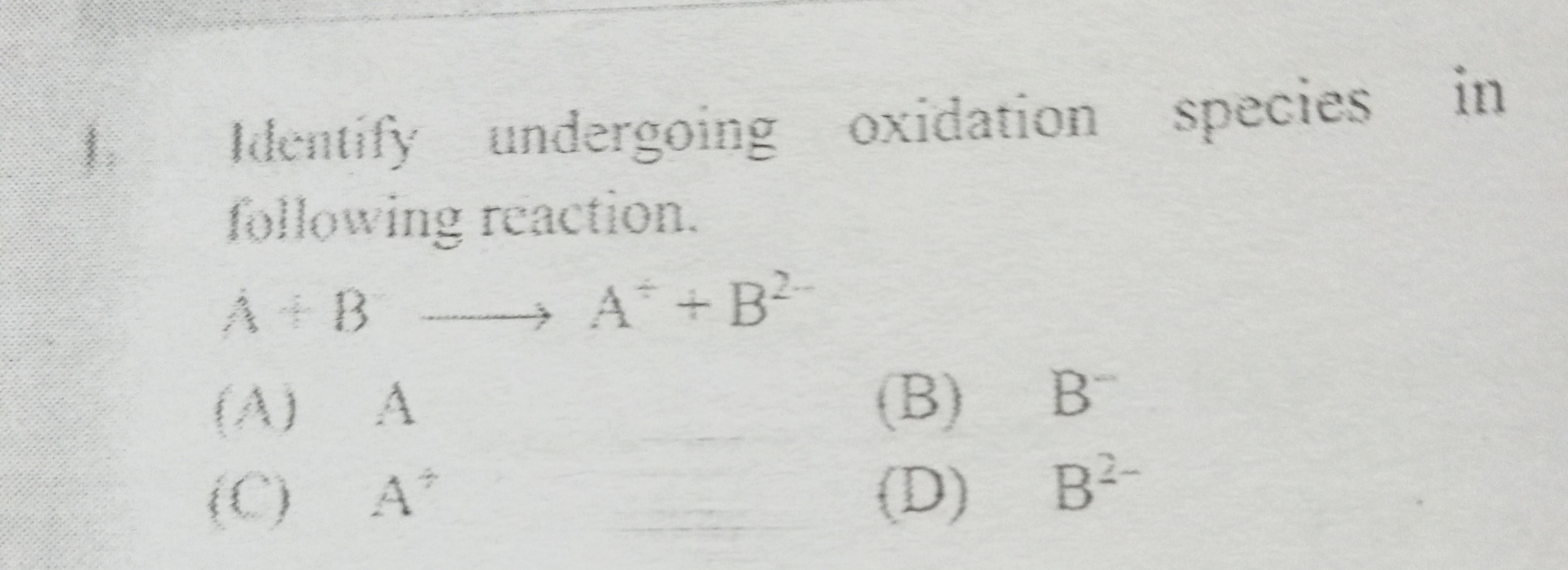
A
A
B
B−
C
A+
D
B2−
Answer
A
Explanation
Solution
For oxidation, the oxidation number increases. Here, element A goes from 0 in A to +1 in A⁺, meaning it has lost an electron. Hence, A is the species undergoing oxidation.
