Question
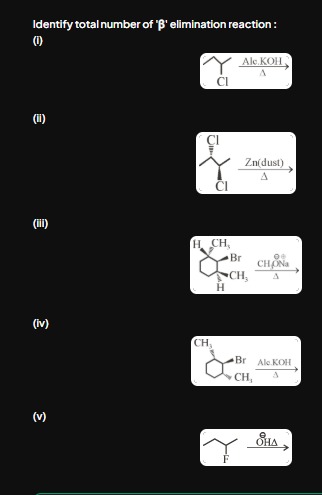
Question: Identify total number of '$\beta$' elimination reaction: (i) (ii) (iii) (iv) (v) ...
Identify total number of 'β' elimination reaction: (i) (ii) (iii) (iv) (v)
4
Solution
A β-elimination reaction (also known as 1,2-elimination) involves the removal of two groups from adjacent carbon atoms, leading to the formation of a double bond. Typically, one group is a leaving group (e.g., halide) from the α-carbon and the other is a hydrogen from the β-carbon.
Let's analyze each reaction:
(i) Reaction (i): The substrate is 2-chloro-2-methylpropane (tert-butyl chloride). The reagent is alcoholic KOH and heat. This is a classic dehydrohalogenation reaction. A hydrogen atom from a β-carbon and the chlorine atom from the α-carbon are removed to form 2-methylpropene. This is a β-elimination reaction (E2 mechanism).
(ii) Reaction (ii): The substrate is 2,3-dichlorobutane (a vicinal dihalide). The reagent is zinc dust and heat. This is a dehalogenation reaction where two halogen atoms are removed from adjacent carbons, forming a double bond (2-butene). This is a type of 1,2-elimination, hence a β-elimination reaction.
(iii) Reaction (iii): The substrate is 1-bromo-1,2-dimethylcyclohexane. The bromine at C1 is in an axial position. The β-carbon C6 has an axial hydrogen which is anti-periplanar to the axial bromine at C1. The reagent, sodium methoxide (CH3O−Na+), is a strong base that promotes E2 elimination. Elimination of the axial hydrogen from C6 and the axial bromine from C1 will occur, forming 1,2-dimethylcyclohexene. This is a β-elimination reaction.
(iv) Reaction (iv): The substrate is 1-bromo-1,2-dimethylcyclohexane. The bromine at C1 is in an equatorial position. For an E2 elimination to occur, the β-hydrogen must be anti-periplanar to the leaving group.
- At C2, the hydrogen is axial, which is not anti-periplanar to the equatorial bromine.
- At C6, neither the axial nor the equatorial hydrogen is anti-periplanar to the equatorial bromine.
Since the required anti-periplanar geometry cannot be achieved in this conformation, E2 elimination is not possible. Therefore, this is not a β-elimination reaction under typical E2 conditions.
(v) Reaction (v): The substrate is 1-fluoro-2-methylpropane (isobutyl fluoride). The reagent is hydroxide ion (OH−) and heat. Although fluorine is a poor leaving group, with a strong base and high temperature, dehydrohalogenation (a β-elimination) can occur, forming 2-methylpropene. This is a β-elimination reaction.
Conclusion: Reactions (i), (ii), (iii), and (v) are β-elimination reactions. Reaction (iv) is not. Total number of β-elimination reactions = 4.
