Question
Question: Identify the unsaturated compound from the following (a)Propane (b)Propene (c)Propyne (d)C...
Identify the unsaturated compound from the following
(a)Propane
(b)Propene
(c)Propyne
(d)Chloropropane
(1)(a) and (b)
(2)(b) and (d)
(3)(c) and (d)
(4)(b) and (c)
Solution
Saturated hydrocarbons contain carbon-carbon single bonds whereas unsaturated hydrocarbons contain carbon-carbon double bonds or triple bonds. A single bond includes a sigma bond and a double bond includes both sigma and pi bonds.
Complete step by step answer:
a.Propane
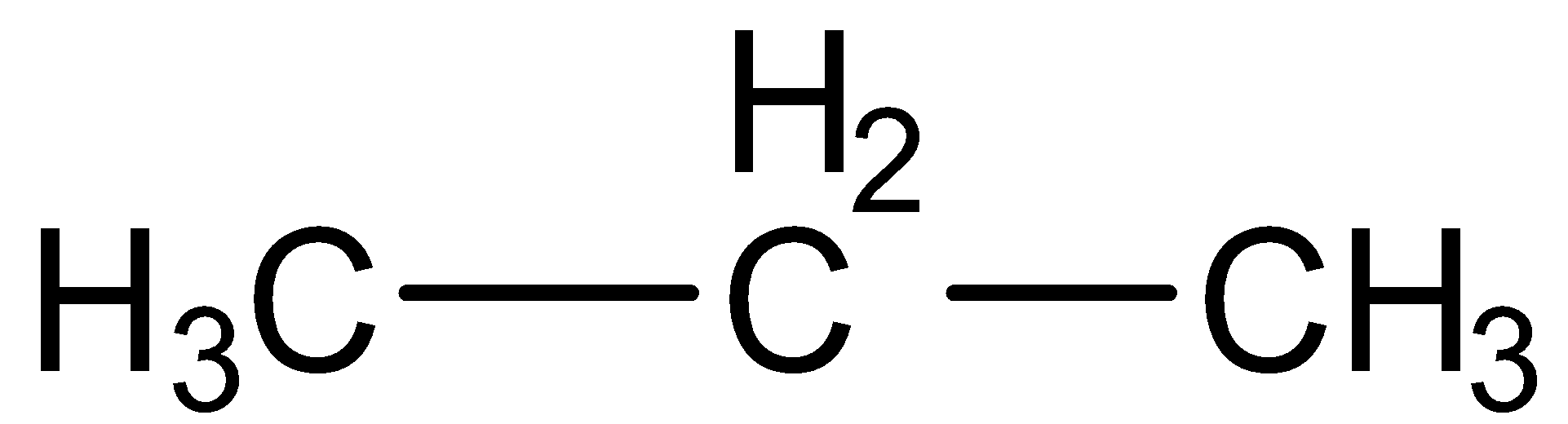
Here carbon-carbon single bond present, thus propane is a saturated hydrocarbon.
b.Propene
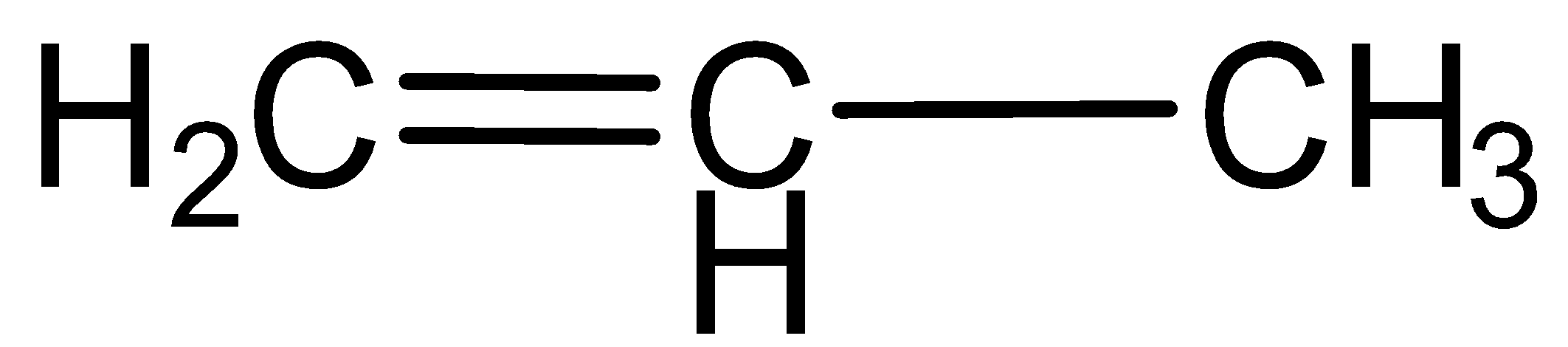
Here hydrocarbons contain carbon- carbon double bonds, which indicate that this is an unsaturated hydrocarbon.
c.Propyne
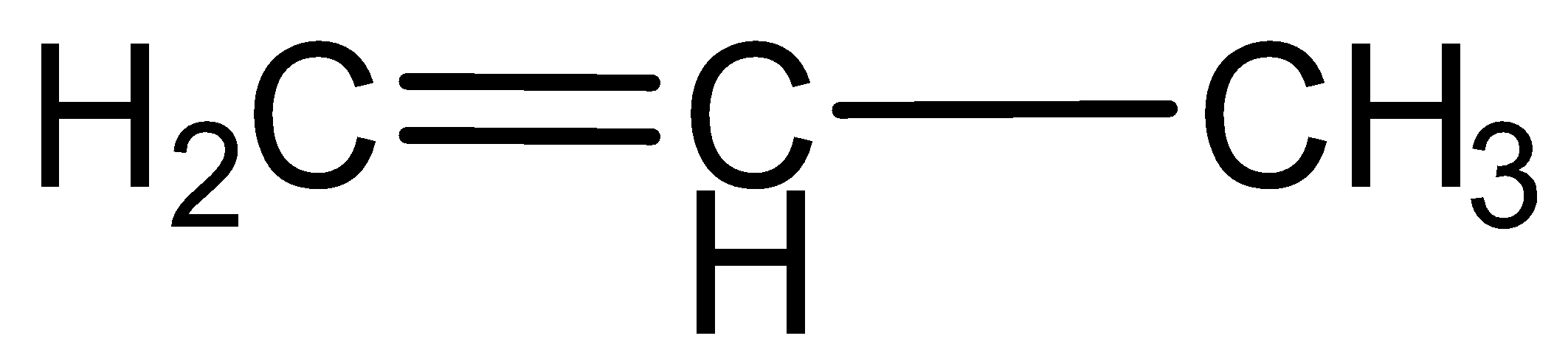
Here carbon- carbon triple bond is present, this is also an unsaturated hydrocarbon.
d.Chloropropane
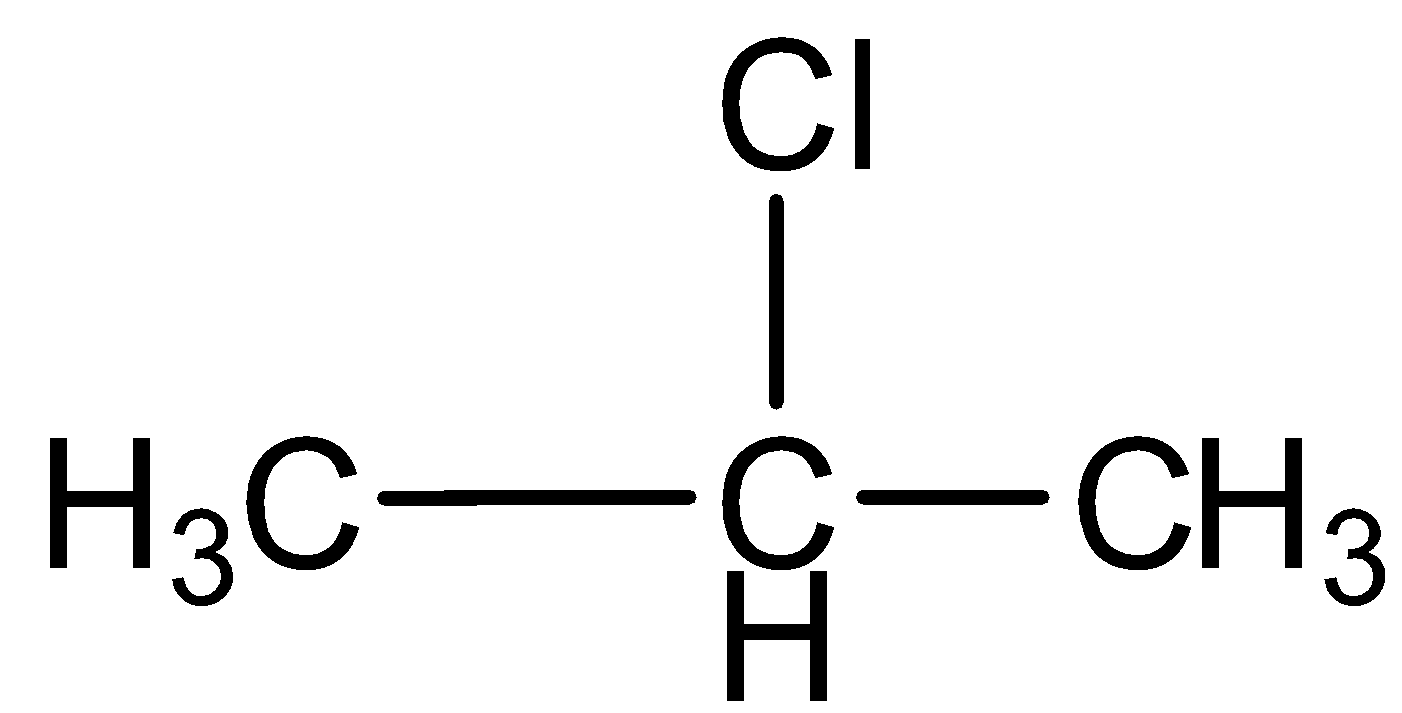
This compound contains a carbon- carbon single bond, so the compound is saturated hydrocarbon.
Therefore, the correct option is (4) (b) and (c).
Additional information:
The structural formulas of alkanes contain four type of carbon atoms:
(1)A carbon atom which is attached to one another is primary carbon.
(2)A carbon atom which is attached to the other two carbon atoms is called secondary carbon.
(3)A carbon atom which is attached to the other three carbon atoms is called tertiary carbon.
(4)A carbon atom which is attached to other four carbon atoms is called quaternary carbon.
Note: The hydrogen atoms in organic compounds may be classified as primary, secondary, tertiary, allylic or benzylic. There are a total six primary hydrogens and two secondary hydrogens in propane; the ratio of primary to secondary hydrogen in propane is 6/2 or 3/1.There is a degree of selectivity in hydrogen abstraction, secondary hydrogen is abstract faster than primary hydrogen.
