Question
Question: Identify the T-shaped molecule. (A)\(B{{F}_{3}}\) (B)\(N{{H}_{3}}\) (C)\(N{{F}_{3}}\) (D)...
Identify the T-shaped molecule.
(A)BF3
(B)NH3
(C)NF3
(D)ClF3
(E)PCl3
Solution
The VSEPR model can be used to determine the shape of a molecule. The valence shell electron pair repulsion theory states that the molecules around the central atom will arrange in such a way that there is minimum repulsion between valence electron pairs of the atom.
Complete answer:
The VSEPR model of arrangement helps in increasing the stability of the molecule and decreasing its energy.
The repulsion between the electron pair increases in the following order
Bond pair-bond pair < bond pair-lone pair < lone pair-lone pair
Now, the formula AXnEm can be used while applying the VSEPR theory to represent the number of electron pairs around a central atom.
Where the central atom is represented by A, ligand bonded to the central atom is represented by X, and lone pairs are represented by E.
Subscripts n and m represent the number of ligands and the number of lone pairs.
Steric Number = n + m
Now, the molecular geometry of the compound according to the steric number and the AXnEm formula is
| STERIC NUMBER | AXnEm | MOLECULAR GEOMETRY |
|---|---|---|
| 2 | AX2E0 | Linear |
| 3 | AX3E0 | Trigonal Planar |
| 3 | AX2E1 | Bent |
| 4 | AX4E0 | Tetrahedral |
| 4 | AX3E1 | Trigonal Pyramidal |
| 4 | AX2E2 | Bent |
| 5 | AX5E0 | Trigonal Bipyramidal |
| 5 | AX4E1 | Seesaw |
| 5 | AX3E2 | T-Shaped |
| 5 | AX2E3 | Linear |
From this table we can see that the T-shape molecules are of type AX3E2.
AX3E2 molecule is bonded to 3 ligands and has 2 lone pairs.
This means that it has 7 electrons in its outermost shell, 3 from the bond pairs, and 4 from the lone pairs.
Since halides have 7 electrons in their outermost shell, the t-shape molecule will be ClF3.
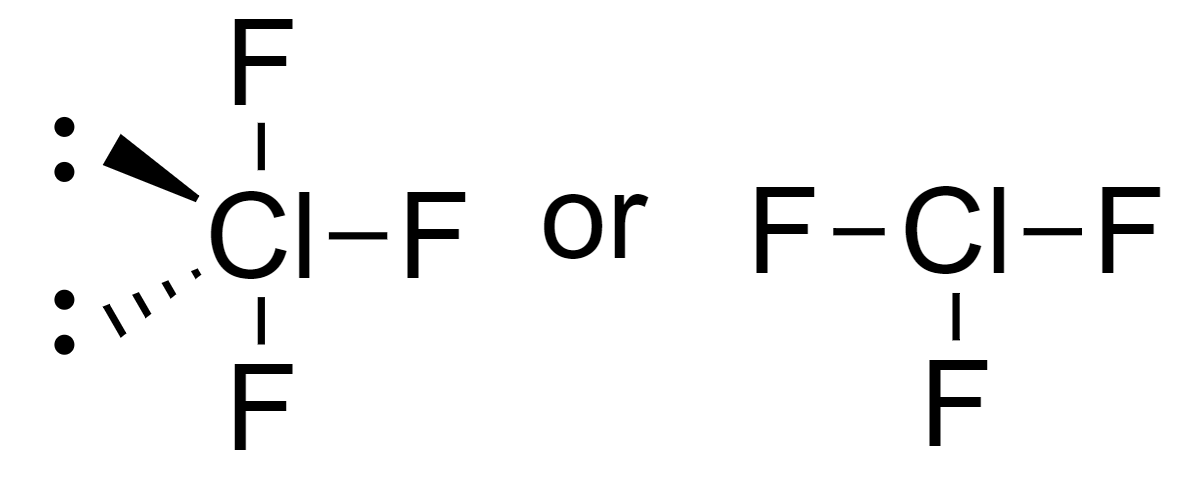
So, the T-shaped molecule is option (D) ClF3.
Note:
It should be noted that the VSEPR theory does not explain the molecular geometry and shapes of the isoelectronic species. It also does not properly explain the molecules of transition metals. It does not consider the inactive lone pairs and the size of the ligand attached to the central atom.
