Question
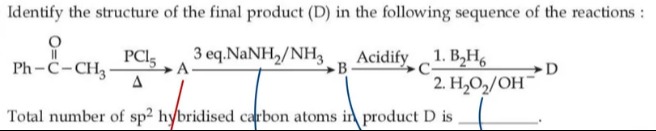
Question: Total number of $sp^2$ hybridised carbon atoms in product D is _______....
Total number of sp2 hybridised carbon atoms in product D is _______.
7
Solution
Solution:
-
Step 1 – Formation of Intermediate A:
PhCOCH3PCl5ΔPhCCl2CH3.
Acetophenone, PhCOCH3, reacts with PCl5 under heating to convert the carbonyl group into a geminal dichloride. Thus, -
Step 2 – Formation of Intermediate B:
PhCCl2CH33 eq. NaNH2/NH3PhC≡CH.
Treatment of PhCCl2CH3 with 3 equivalents of NaNH2 in NH3 causes double dehydrohalogenation. This elimination removes two molecules of HCl to generate a triple bond, forming phenylacetylene (which, in its anionic form, is later finally protonated). -
Step 3 – Formation of Intermediate C:
Acidification of the reaction mixture protonates the acetylide ion (if present) to give phenylacetylene, PhC≡CH. -
Step 4 – Formation of Final Product D:
Phenylacetylene is then subjected to hydroboration–oxidation:- First, reaction with B2H6, and then
- Oxidation with H2O2 in the presence of OH− converts the alkyne to an enol, which tautomerizes to give the methyl ketone, acetophenone (PhCOCH3).
Thus, the final product D is acetophenone.
Counting sp² Hybridized Carbon Atoms in Product D (Acetophenone):
- Benzene ring: 6 sp² hybridized carbons
- Carbonyl carbon: 1 sp² hybridized carbon
