Question
Question: Identify the products of the reaction: 
A) CH3CHO
B) CH3CH2OH
C) CH3COOH
D) CH3OH
Solution
The given reactant to us is Acetylene. It is an alkyne with two carbon atoms and a triple bond. It has a formula C2H2 . The reagent given to us is a strong oxidising agent Potassium Dichromate. Potassium Dichromate on reaction with Sulphuric acid, gives nascent oxygen which causes oxidation.
Complete answer:
Acetylene, in reaction with Strong oxidising agent like Potassium dichromate, converts it into acetic acid. But note that this reaction cannot occur directly. First, we’ll have to change the Acetylene into Aldehyde and then on further oxidation it changes into carboxylic acid.
Conversion of acetylene into aldehyde occurs in presence of 40%H2SO4 and 1%HgSO4 at 60∘C. The reaction for the conversion of Acetylene into aldehyde can be given as:
C2H2H2SO4HgSO4CH3CHO
Here, acetylene gets converted into acetaldehyde. Acetaldehyde is a two-carbon aldehyde, has a Carbonyl Group and no other C-C multiple bonds present.
Further on reaction with Oxidising agents like Potassium Dichromate, it gets converted into acetic acid.
CH3CHO[O]CH3COOH
The overall reaction can be shown as:
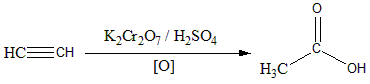
Hence the correct answer is Option (C)
Option (A) would be incorrect because Potassium Dichromate is a strong oxidising agent, and the reaction will proceed further until no further oxidation can take place. Hence it will form the most oxidised product, which in this case is the Carboxylic acid. If mild oxidising agents like PCC were used, then the reaction would stop at the formation of the first oxidised product; aldehyde.
Hence the correct answer is Option (C).
Note:
Acetylene and acetic acid are the common names known for these compounds. The IUPAC names of these compounds are Ethyne (acetylene) and Ethanoic Acid (acetic acid). The major use of acetic acid in households is in the form of Vinegar, which is the diluted form of it.
