Question
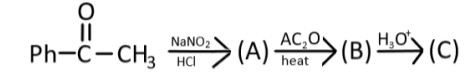
Question: Identify the product (C) in the given reaction;  in the given reaction;
(1) 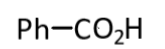
(2) 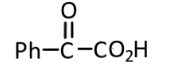
(3) 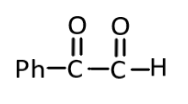
(4) 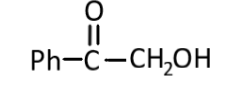
Solution
The starting reactant has a carbonyl group, and the name of the functional group is a ketone (acetophenone), with a phenyl group, and if observed the groups are nitrous acid (HONO), acetic anhydride, and the last step is hydrolysis.
Complete step-by-step answer: So, here we have the first reactant is acetophenone, which is reacting withNaNO2/ HCl, this group will give a nitrous acid (HONO), which will attack the acetophenone and form an oxime derivative. Following is the reaction observed in the formation of product ‘A’
NaNO2 + HCl→HNO2
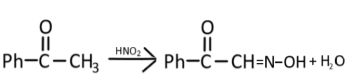
Ph−C||O−CH=N−OH is the product (A) that is formed, now this oxime derivative will react with acetic anhydride and remove H2O molecules from the oxime derivative compound. The following reaction will take place:
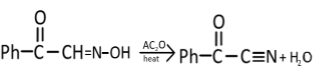
Now, in the last step, the nitrile group will get hydrolyzed (H3O+) and form a carboxylic acid derivative:
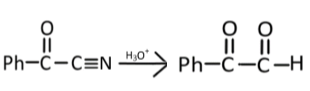
Hence, the correct answer is option(3).
Note: : In the reaction of acetophenone with (HONO), there are two possible products of ‘A’, both have a similar molecular formula, 1st oxime derivative and the other with the tautomerization of oxime (Ph−C||O−CH2−N=O), but we have used the oxime derivative as in the next step of dehydration the oxime derivative will be more easily lose H2O molecules and form the corresponding product with a nitrile group.
