Question
Question: Identify the product A in the given reaction 
A. 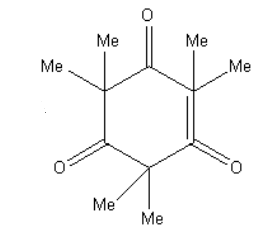
B. 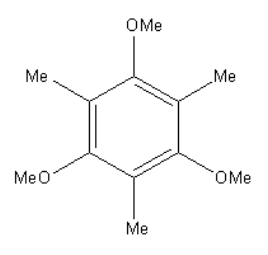
C. 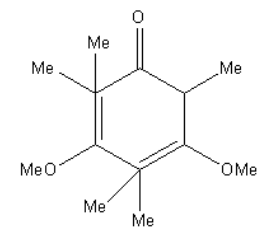
D. 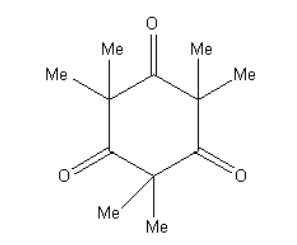
Solution
The base is used to abstract the proton which initiates the substitution of hydrogen by a methyl group. The phenol can show keto-enol tautomerism to generate the hydroxyl group again and the substitution with the methyl group. So, all six hydrogens can be replaced with methyl groups.
Complete step by step answer:
The sodium hydroxide is a base that can abstract the proton from phenol. The hydrogen attached with oxygen is most acidic, so base abstract that proton and form phenoxide ion.
The reaction is as follows:
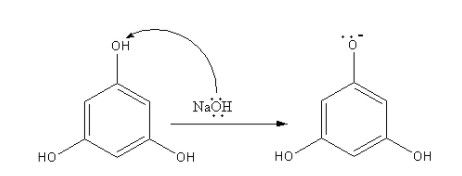
The negative charge of oxygen of phenoxide is delocalised to give a nucleophilic carbon which attacks the methyl iodide to give methyl substituted product.
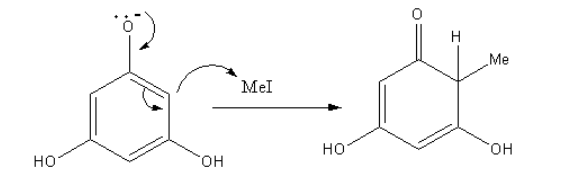
The obtained product has the keto group. It show keto-enol tautomerism as follows:
By the tautomerism keto functional group converts into the hydroxyl group.
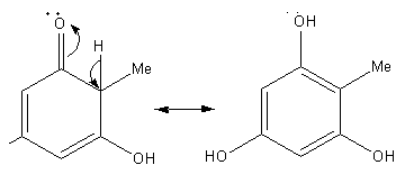
Again the base abstract hydrogen then delocalization of charge generates the negatively charged carbon which attacks methyl iodide to give the product having two methyl groups.
As the base present in excess so, the hydrogen will be abstracted and the final product will contain methyl in place of hydrogens so, the final product is as follows:
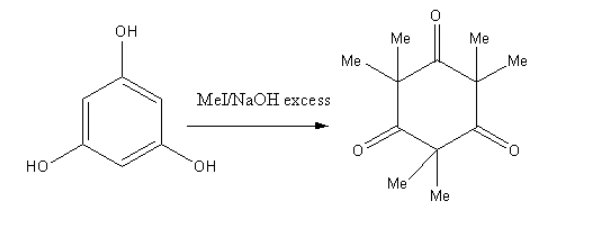
**Therefore, option (D) is correct.
Note: **
The reaction of phenol with methyl iodide in presence of base is an example of an electrophilic substitution reaction. The form having hydroxyl is known as enol and the form having ketone group is known as keto form. The enol form of phenol is more stable than the keto form due to aromatization.
