Question
Question: Identify the product A: 
A. 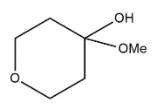
B. 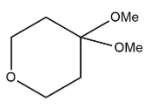
C. 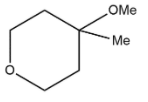
D. 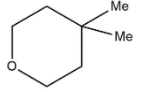
Solution
Trimethyl orthoformate is an organic compound which is represented by the formula HC(OCH3)3. It is generally a colorless liquid and used as a reagent in organic synthesis for the formation of methyl esters.
Complete step by step answer:
The reaction can be explained by the following mechanism:
In the first step HC(OCH3)3 reacts with HOH/H+ and the reaction can be shown as:
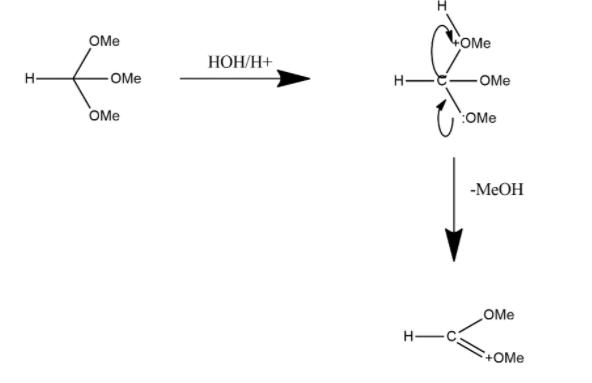
In this one CH3 group is lost in the term of MeOH and the lone pair present on that group gets transferred to other ion and forms double bond product and in the next step this product gets reacted with water molecules and reaction can be shown as:
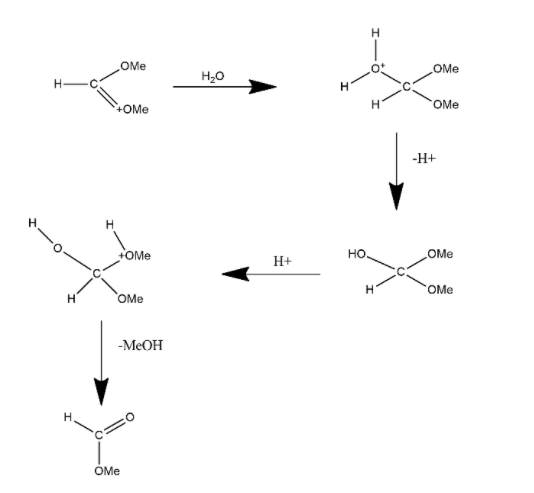
This water molecule get attached with carbon atom and then loss of proton takes place and after that proton is added to the compound again by which it again loses its one methyl group in the form of MeOH and the product form during this reaction is aldehyde in nature i.e. it forms aldehydic group.
In this way it forms this product which is already given in the reaction and other product can be defined by the following mechanism when reactant given is react with H+ and then react with MeOH and form the following product which further get reacted with H+ and form water molecule which further loses and form double bond and this compound further reacted with MeOH and forms the product given in the option B.
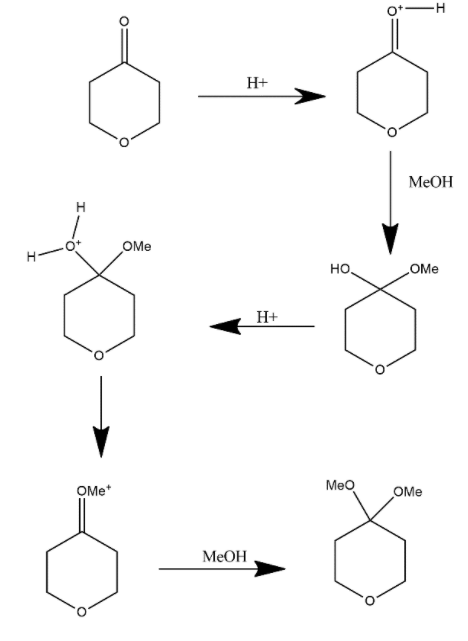
Hence we can say that option B is the correct answer.
Note: Trimethyl orthoformate can be prepared by the reaction between chloroform and sodium methoxide under the process named as williamson ether synthesis. If in the given reaction reactant is CH(OEt)3 is this instead of HC(OCH3)3 then major product will be ortho directing group.
