Question
Question: Identify the pair of alkanes which can exhibit chain isomerism and have a neo isomer respectively? ...
Identify the pair of alkanes which can exhibit chain isomerism and have a neo isomer respectively?
A. C3H8, C4H10
B. C4H10, C5H12
C. C5H12, C6H14
D. C6H14,C7H16
Solution
The general configuration of alkanes is CnH2n+2, where n is the natural number. Draw the isomers of the given alkanes to check whether they show chain and neo isomerism or not. Also, the minimum requirement of carbon atoms for this isomerism should be known, which is 4 in chain and 5 in neo isomerism.
Complete answer:
Let us first discuss what chain and neo isomers are:
Chain isomers- The carbon compounds with the same molecular formula but have different atomic arrangements, the one with branches. The minimum number of carbon atoms required for chain isomerism are 4.
Neo isomers- In this, a carbon atom is attached to four other carbon atoms. Neo-isomers are the type of chain isomers only. The minimum number of carbon atoms required for neo isomers are 5.
Let us discuss the options one-by-one along with their chain and neo isomers:
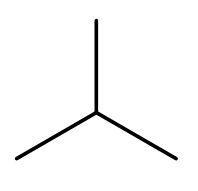
A) C3H8, C4H10- The compound C3H8 is propane. As, the number of carbon atoms are less than 4, so it cannot have chain isomerism. C4H10 is butane. It has four carbon atoms, so it can form chain isomers. But, it cannot form neo isomers. The only isomer of butane is 2-methyl propane. The only isomer is
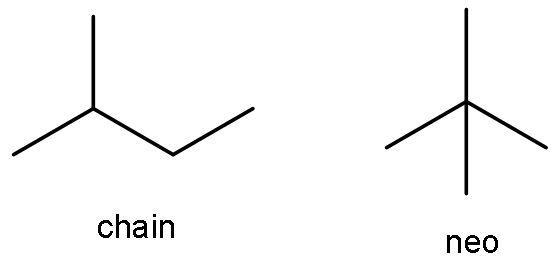
B) C4H10, C5H12- C4H10is butane. It has four carbon atoms, so it can form chain isomers. But, it cannot form neo isomers. As given in the above part the only isomer of butane is 2-methyl propane.
The compound C5H12 is pentane. It has both chain and neo-isomer, as it has 5 carbon atoms. The chain isomer is 2-methyl butane. The neo isomer is 2,2-dimethylpropane.
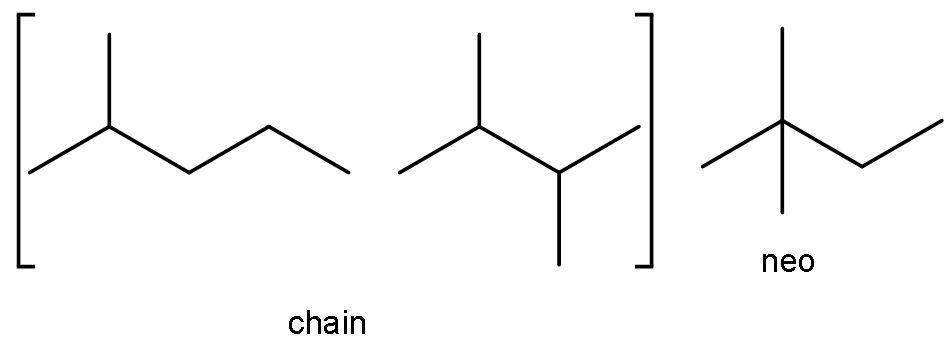
C)C5H12, C6H14: As discussed in the above solution part, the compound C5H12 is pentane. It has both chain and neo-isomer. The chain isomer is 2-methyl butane. The neo isomer is 2, 2-dimethylpropane.
The compound C6H14 is hexane. It has both chain and neo-isomer, as it has more than 5 carbon atoms. The chain isomers are 2-methyl pentane and 2,3-dimethyl butane. The neo isomers are 2,2-dimethylbutane.
D)C6H14,C7H16: As given in the above solution part we know that hexane has both chain and neo-isomers. The chain isomers are 2-methyl pentane and 2,3-dimethyl butane. The neo isomers are 2, 2-dimethylbutane.
The compound C7H16 is heptane. It has both chain and neo-isomer, as it has more than 5 carbon atoms. The chain isomers are 2-methyl hexane, 2,3-dimethyl pentane and 2,4-dimethyl pentane.
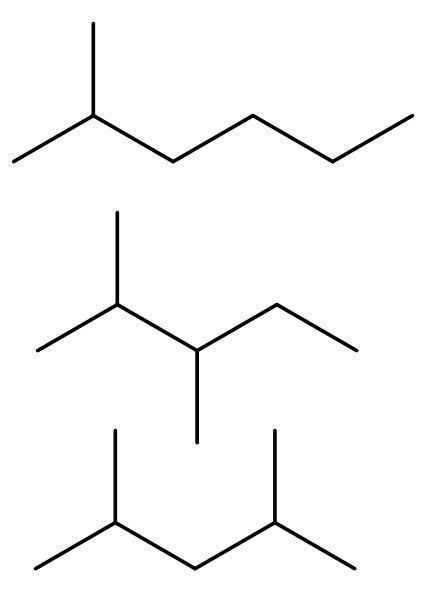
The neo isomers are 2,2-dimethyl-3-methyl butane, 2,2-dimethyl pentane and 3,3-dimethyl pentane.
C5H12, C6H14 and C6H14,C7H16 are the pair of alkanes which can exhibit chain isomerism and has a neo isomer respectively.
The correct options are (c) and (d).
Note:
As the branching in the carbon chain increases, the surface area decreases and vander waal forces of attraction decrease. The force of attraction decreases, thus, the boiling point of alkane is also decreased. The neo isomers have less boiling point than chain isomers.
