Question
Question: Identify the odd one out of the following: A.Benzene (\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{...
Identify the odd one out of the following:
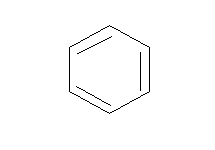
A.Benzene (C6H6)
B.Cyclobutane (C4H8)

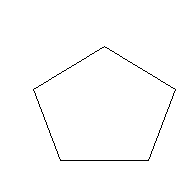
C.Cyclopentane (C5H10)
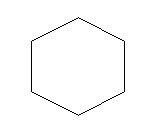
D.Cyclohexane (C6H10)
Solution
Organic cyclic compounds are divided into three categories:
-Aromatic – are cyclic, conjugated and have (4n+2)pi electrons
-Anti-aromatic – are cyclic, conjugated and have (4n) pi electrons
-Non-Aromatic – are those compounds which do not fall under the aromatic or antiaromatic category.
Complete step by step answer:
Benzene: Benzene is a cyclic compound having conjugation (alternative single and double bond). There are 6 pi electrons in the benzene, thus following the (4n+2) pi-electron rule where n= 1.
This means benzene is an aromatic compound.
Cyclobutane: Cyclobutane is a cyclic compound but it does not have any conjugation and no pi electrons. Therefore it has neither (4n+2) pi electrons nor 4n pi electrons. Thus it falls under the category of Non-aromatic compounds
Cyclopentane: Cyclopentane is a cyclic molecule but has no conjugation and pi electrons are also absent. Since it does not satisfy the conditions of either aromatic or ant-aromatic, it is a non-aromatic compound.
Cyclohexane: Cyclohexane is again a cyclic compound but like cyclobutane and cyclopentane it does not satisfy the condition of either aromatic or antiaromatic and hence comes under non-aromatic compounds.
The above points indicated that among all the given four compounds, benzene is the only one which is aromatic and the rest of the three are non-aromatic compounds.
Hence, the correct answer is A.
Note:
Since we have talked about conjugation as a necessary condition for aromatic and antiaromatic compounds, it will be good to know what conjugation is. A conjugated system is a system of connected p-orbitals with delocalised electrons in a molecule. Conjugation is responsible for extra stability of the molecule.
