Question
Question: Identify the number of reactions that correctly match with their major products....
Identify the number of reactions that correctly match with their major products.
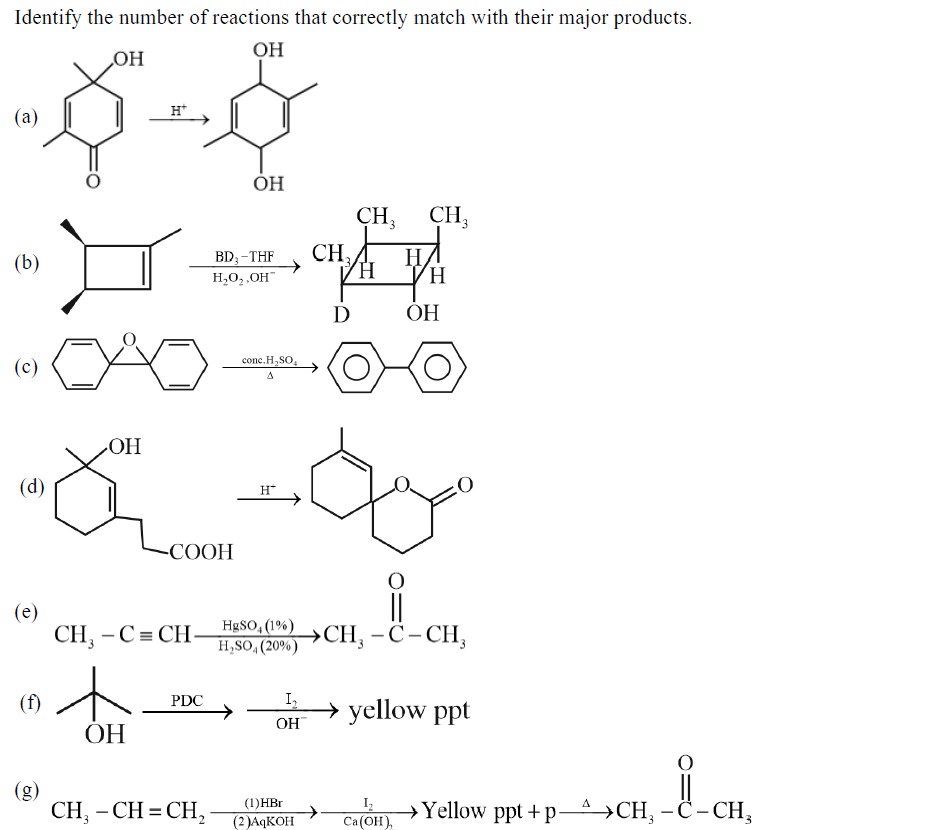
(a)
H+
(b)
BD3−THF H2O2,OH−
(c)
conc.H2SO4 Δ
(d)
H+
(e)
CH3−C≡CH HgSO4(1%)H2SO4(20%) CH3−C∣∣−CH3
(f)
PDC I2OH− yellow ppt
(g)
CH3−CH=CH2 (1)HBr(2)AqKOH I2Ca(OH)2 Yellow ppt + p Δ CH3−C∣∣−CH3
2
Solution
The question asks us to identify the number of reactions that correctly match with their major products. We will analyze each reaction individually.
Reaction (a):
The reactant is 4-hydroxy-4,4-dimethylcyclohexa-2,5-dien-1-one. This is a substituted cyclohexadienone with a tertiary alcohol group at C4.
Under acidic conditions (H+), this compound undergoes a dienone-phenol rearrangement. The mechanism involves protonation of the carbonyl oxygen, followed by a 1,2-shift of a methyl group from C4 to C3 (or C5), and subsequent deprotonation to form an aromatic phenol.
The expected product from 4-hydroxy-4,4-dimethylcyclohexa-2,5-dien-1-one is 2,4-dimethylphenol.
The product shown is 2,5-dimethylbenzene-1,4-diol (a hydroquinone derivative). This is incorrect. The rearrangement of a dienone typically yields a phenol, not a hydroquinone.
Therefore, reaction (a) is incorrect.
Reaction (b):
The reactant is 1,2-dimethylcyclobutene. The reaction is hydroboration-oxidation using BD3-THF followed by H2O2, OH−.
Hydroboration-oxidation is a syn-addition of H (or D) and OH across the double bond, following anti-Markovnikov's rule.
In 1,2-dimethylcyclobutene, both carbons of the double bond (C1 and C2) are equally substituted (each has one methyl group and is part of the ring). So, D can add to either C1 or C2, and OH to the other.
The addition is syn, meaning D and OH will be on the same face of the cyclobutene ring.
The starting material 1,2-dimethylcyclobutene is usually considered to have cis methyl groups unless specified otherwise. If the methyl groups are cis, then after syn addition of D and OH, the resulting product should also have cis methyl groups relative to each other, and D and OH should be cis.
The product shown has the two methyl groups trans to each other, and D and OH are cis. This implies that the starting material must have been trans-1,2-dimethylcyclobutene, which is less common or specifically drawn. Assuming the standard cis-1,2-dimethylcyclobutene, the product's stereochemistry is incorrect.
Therefore, reaction (b) is incorrect.
Reaction (c):
The reactant is diphenyl ether (Ph-O-Ph). The reagents are concentrated H2SO4 and heat (Δ).
Concentrated H2SO4 and heat are strong acidic and dehydrating conditions. Diphenyl ether is an inert compound. Under these conditions, it is known to undergo decomposition or cleavage, for example, producing phenol and benzene.
The product shown is biphenyl (Ph-Ph). Biphenyl is typically formed by reactions like the Ullmann reaction or oxidative coupling of benzene, not by rearrangement or cleavage of diphenyl ether under these conditions. The Fries rearrangement applies to aryl esters, not ethers.
Therefore, reaction (c) is incorrect.
Reaction (d):
The reactant is 1-hydroxy-1-methyl-2-(2-carboxyethyl)cyclohex-2-ene. It has a tertiary alcohol at C1, a methyl group at C1, a double bond between C2 and C3, and a carboxylic acid group attached to C2 via a two-carbon chain.
Under acidic conditions (H+), the tertiary alcohol can be protonated and leave as water, forming a tertiary carbocation at C1.
This carbocation can then be attacked intramolecularly by the carboxylic acid group to form a lactone.
Let's trace the atoms for the lactone formation:
-
Protonation of -OH at C1 and loss of H2O forms a carbocation at C1.
-
The oxygen of the carboxylic acid group (-COOH) attacks the carbocation at C1. The chain from C2 is -CH2-CH2-COOH.
The atoms involved in the lactone ring formation would be:
C1 (from cyclohexene) - O (from COOH) - C(=O) (from COOH) - CH2 - CH2 - C2 (from cyclohexene) - C1.
This forms a 7-membered lactone ring (1-O-CO-CH2-CH2-C2-C1).
However, the product shown is a 6-membered lactone ring fused at the spiro carbon (C1).
The product structure indicates that the original C1, C2, and the -CH2CH2COOH chain form the lactone.
Let's count the atoms in the lactone ring of the product: The spiro carbon (C1) is shared. The lactone ring contains C1, C2, and the atoms of the side chain.
The lactone ring is C1-O-C(=O)-CH2-CH2-C(ring)-C1. This is a 6-membered ring.
The product shown is 1'-methyl-3,4,5,6-tetrahydrospiro[furan-2,1'-cyclohexene]-2'-one. This structure corresponds to an intramolecular esterification where the OH at C1 reacts with the COOH, forming a 6-membered lactone by a specific cyclization.
The reaction is a known lactonization. The carbocation at C1 is attacked by the hydroxyl oxygen of the carboxylic acid (after tautomerization to the enol form if needed, or direct attack by the oxygen of the -OH of the COOH group).
The product shown is consistent with the intramolecular esterification of the tertiary alcohol with the carboxylic acid, leading to a 6-membered lactone ring.
Therefore, reaction (d) is correct.
Reaction (e):
The reactant is propyne (CH3-C≡CH).
The reagents are HgSO4 (1%) and H2SO4 (20%). This is the acid-catalyzed hydration of an alkyne.
Hydration of terminal alkynes follows Markovnikov's rule, adding H to the less substituted carbon and OH to the more substituted carbon.
CH3-C≡CH + H2O HgSO4,H2SO4 CH3-C(OH)=CH2 (enol)
The enol intermediate (prop-1-en-2-ol) immediately tautomerizes to the more stable keto form (propan-2-one or acetone).
CH3-C(OH)=CH2 ⇌ CH3-C(=O)-CH3
The product shown is CH3-C(=O)-CH3 (acetone).
This reaction and product are correctly matched.
Therefore, reaction (e) is correct.
Reaction (f):
The reactant is 2-methylpropan-2-ol (tert-butyl alcohol).
The first reagent is PDC (Pyridinium Dichromate), which is a mild oxidizing agent.
PDC is used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. Tertiary alcohols are generally resistant to oxidation under these conditions.
Tertiary alcohols do not have a hydrogen atom on the carbon bearing the hydroxyl group, which is required for oxidation.
So, 2-methylpropan-2-ol will not be oxidized by PDC.
Even if it were oxidized (e.g., under very harsh conditions leading to C-C bond cleavage), it would not form a compound that gives a yellow precipitate with I2/OH−.
The second step is I2/OH−, which is the iodoform test. The iodoform test gives a yellow precipitate (CHI3) for compounds containing a CH3CO- group or a CH3CH(OH)- group.
Since 2-methylpropan-2-ol is a tertiary alcohol, it cannot be oxidized to a methyl ketone or a secondary alcohol with the required structure for the iodoform test.
Therefore, reaction (f) is incorrect.
Reaction (g):
The reactant is propene (CH3-CH=CH2).
(1) HBr: Addition of HBr to propene follows Markovnikov's rule, forming 2-bromopropane.
CH3-CH=CH2 + HBr → CH3-CH(Br)-CH3 (2-bromopropane)
(2) Aq. KOH: This is a nucleophilic substitution reaction. 2-bromopropane reacts with aqueous KOH to form 2-propanol.
CH3-CH(Br)-CH3 + Aq. KOH → CH3-CH(OH)-CH3 (2-propanol)
Next step: I2/Ca(OH)2 (or I2/OH−): This is the iodoform test.
2-propanol (CH3-CH(OH)-CH3) has the CH3CH(OH)- group, so it gives a positive iodoform test (yellow precipitate of iodoform, CHI3). This part is correct.
The 'p' refers to the product formed after the iodoform test, which is the carboxylate salt (CH3COO−) that upon acidification gives acetic acid.
The final step is Δ (heat) to produce CH3-C(=O)-CH3 (acetone).
The iodoform reaction leads to the formation of iodoform (yellow ppt) and the carboxylate salt. The carbon chain is cleaved.
From 2-propanol, the iodoform reaction yields iodoform (CHI3) and sodium acetate (CH3COONa, if NaOH is used) or calcium acetate (if Ca(OH)2 is used).
The final product shown is acetone (CH3-C(=O)-CH3). Acetone is the starting material for the iodoform reaction if a methyl ketone is used, or it's an intermediate if a secondary alcohol is oxidized.
However, the sequence of reactions is: propene → 2-bromopropane → 2-propanol.
Then, 2-propanol undergoes the iodoform test. The iodoform test is a destructive test that cleaves the carbon chain. It produces iodoform (yellow ppt) and acetate (CH3COO−). It does not produce acetone as the final organic product.
Therefore, reaction (g) is incorrect.
Summary of correct reactions:
(a) Incorrect
(b) Incorrect
(c) Incorrect
(d) Correct
(e) Correct
(f) Incorrect
(g) Incorrect
Number of correctly matched reactions = 2.
