Question
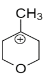
Question: Identify the most stable structure among the following : A.
B.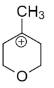
C.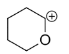
D.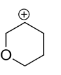
Solution
To solve this question, we should first know about the stability of carbocation. The positive inductive effect increases the stability of carbocation, +I groups donate negative charge density on carbon decreasing the positive charge on the carbocation which makes it more stable whereas the Negative inductive effect decreases the stability of the carbocation.
Complete step by step answer:
A carbon atom having a positive charge and three bonds are called carbocation. A carbon atom has a negative charge and its trivalent is called carbanion.
Inductive effect is the effect in which a sigma electron displaces towards the more electronegative atom which makes one end of the atom positively charged and the other end of the atom becomes negatively charged, that is known as the Inductive effect. It is a permanent effect that is represented by an arrow on the bond of the molecule. The inductive effect is of two types – I effect and + I effect.
The process of delocalizing the electrons in molecules where the bonding cannot be described by just one lewis structure is known as Resonance.
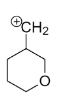
In structure A, the positive charge being far away from the oxygen, It cannot get into close contact with the lone pair possessed by oxygen. So the effect of oxygen destabilizes the +ve charge.
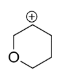
In structure B, again the positive charge is far away from the oxygen, I effect makes it less stable.
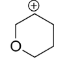
In structure C, The oxygen possesses a lone pair which shows resonance and it goes to the next position which has +ve charge. The lone pair of oxygen stabilizes the carbocation making it the most stable one.
In structure D, the positive charge being far away from the oxygen, It cannot get into close contact with the lone pair possessed by oxygen. So the effect of oxygen destabilizes the +ve charge.
So, Option (C)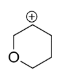 is correct.
is correct.
Note: In Resonance, Every lewis structure contributes to the formation of the target molecule or ion. One should not mistake the lewis structures being the isomers as only the position of delocalized electrons differs. It is also known as mesomerism.
