Question
Question: identify the major product of the reaction 
(a)
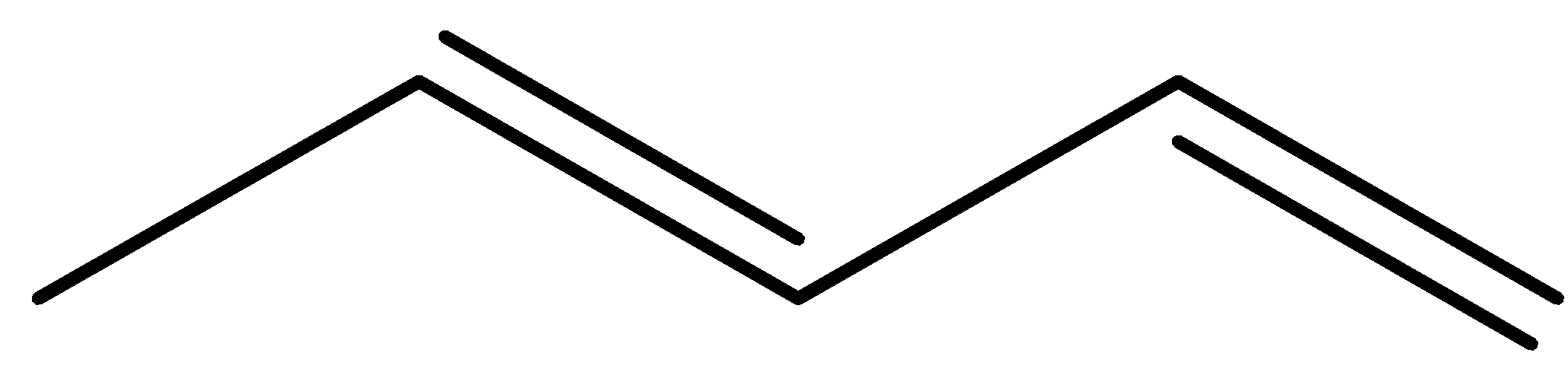
(b)
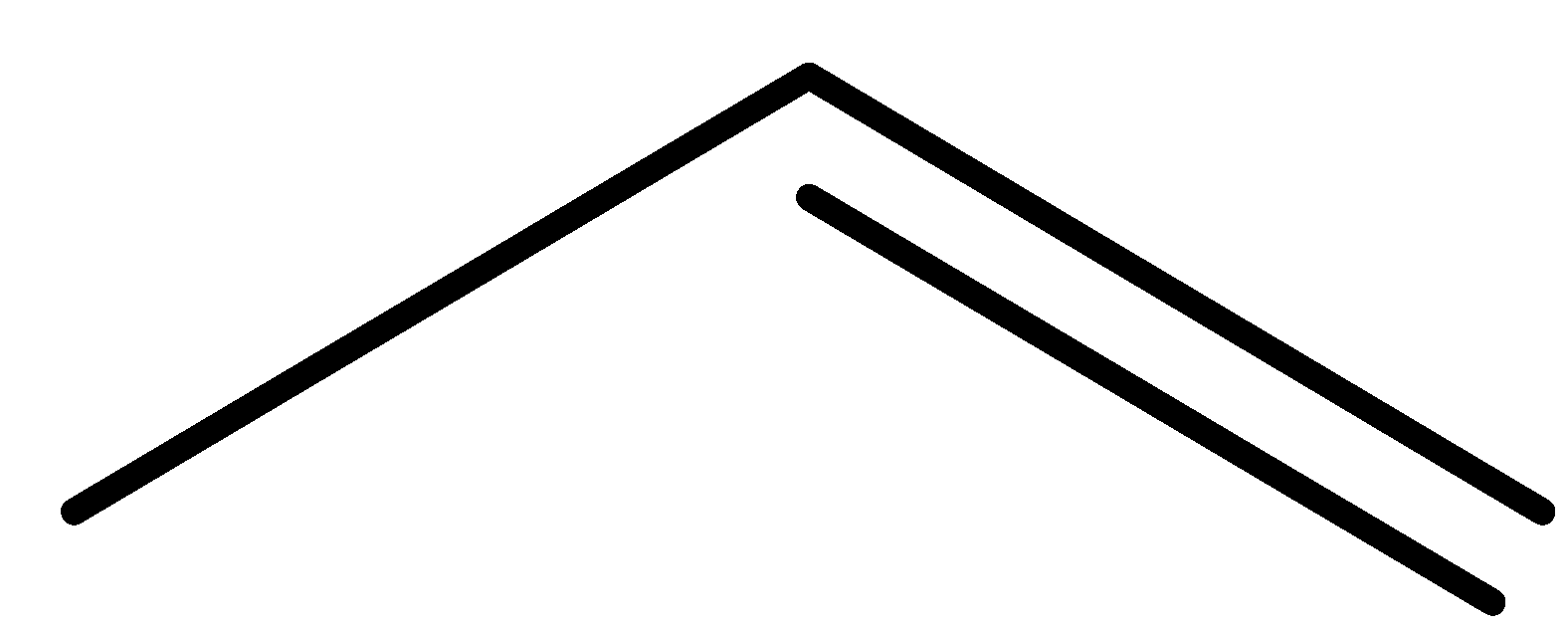
(c)
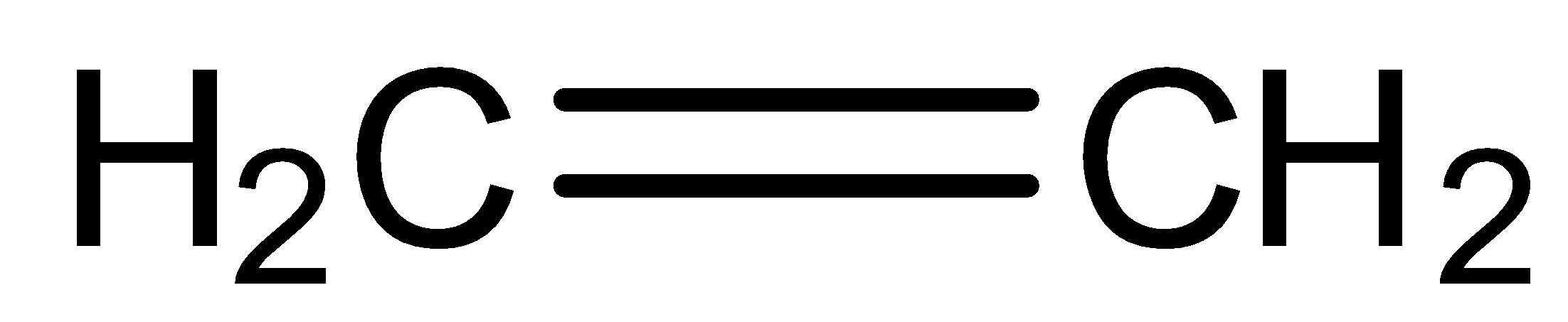
(d)
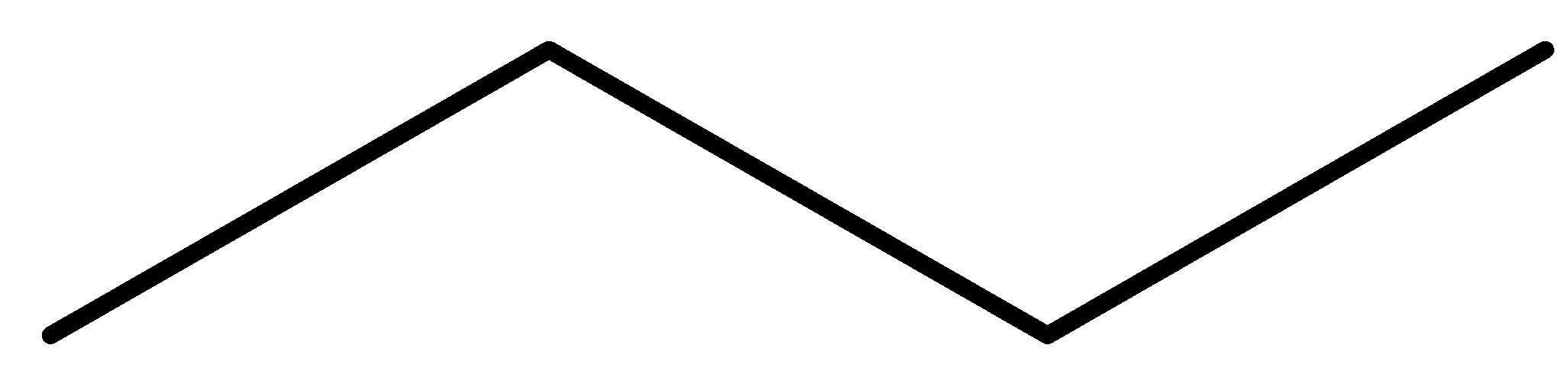
Solution
We know that, amine is an organic compound whose structure is R−NH2. This compound is a derivative of an ammonia molecule. There are three types of amine, primary amine, secondary amine and tertiary amine.
Complete step by step solution:
Let’s discuss types of amine in detail. Primary amine is the amine that is formed by replacing one hydrogen atom of ammonia by an alkyl group.
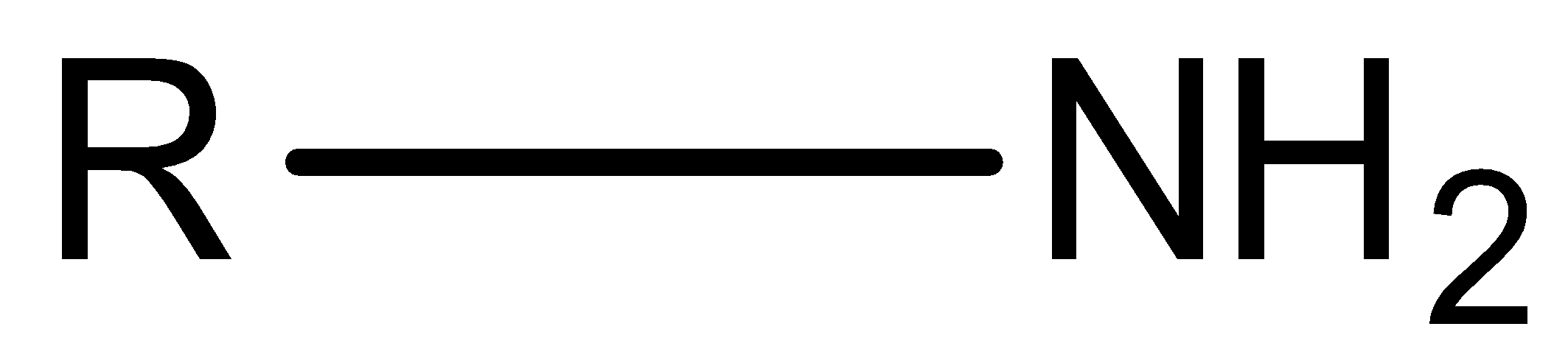
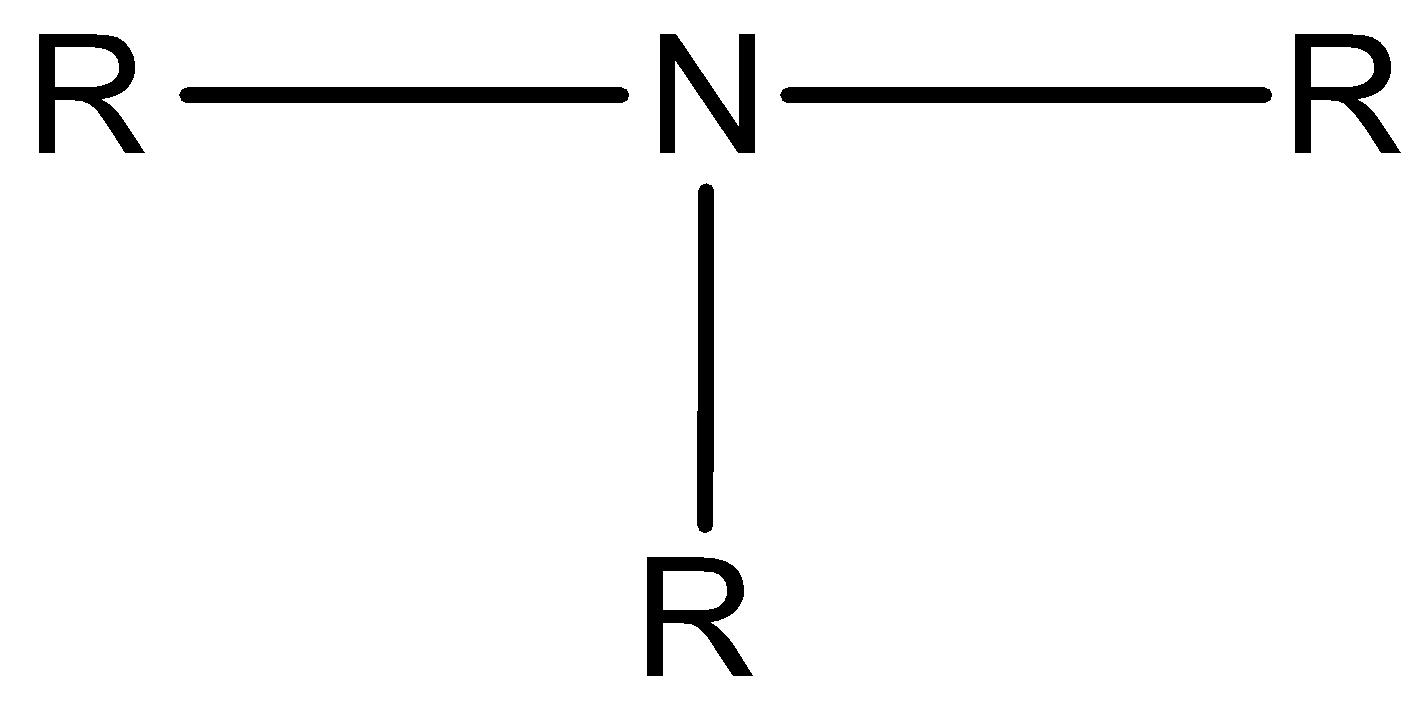
Secondary amine is the amine in which two hydrogen atoms of ammonia are replaced by two alkyl groups.

Tertiary amine is the amine in which all three hydrogen atoms of ammonia are replaced by three alkyl groups.
Now, come to the question. Here, a tertiary amine undergoes a reaction with peroxybenzoic acid (PhCO3H)S. Peroxybenzoic acid is an oxidizing agent. It can supply oxygen atoms with six electrons. The product formed in the reaction is amine oxide. So, the reaction of the given compound with peroxybenzoic acid results,
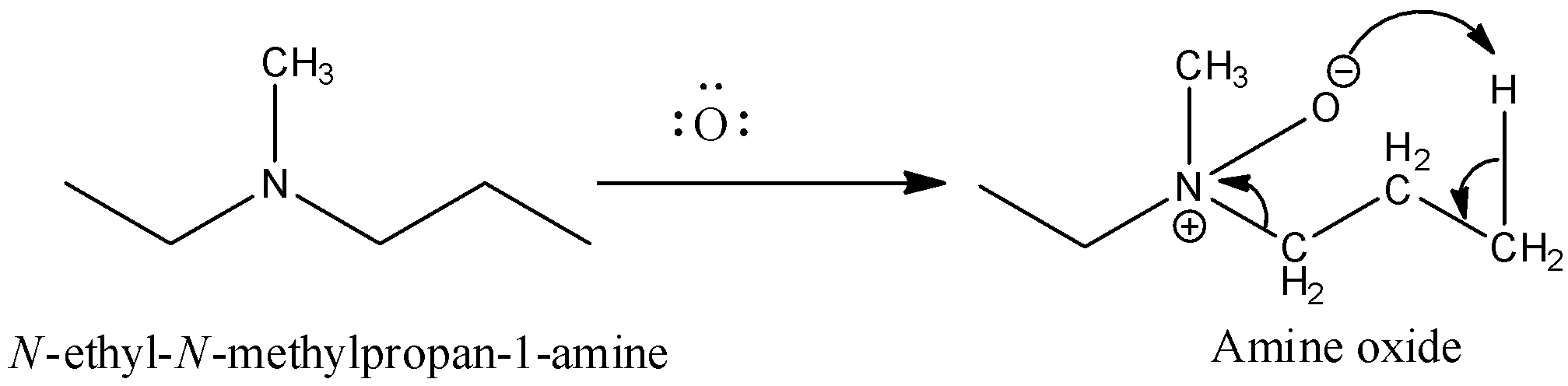
Then the amine oxide is heated. The heating causes decomposition of amine oxide and results in alkene.
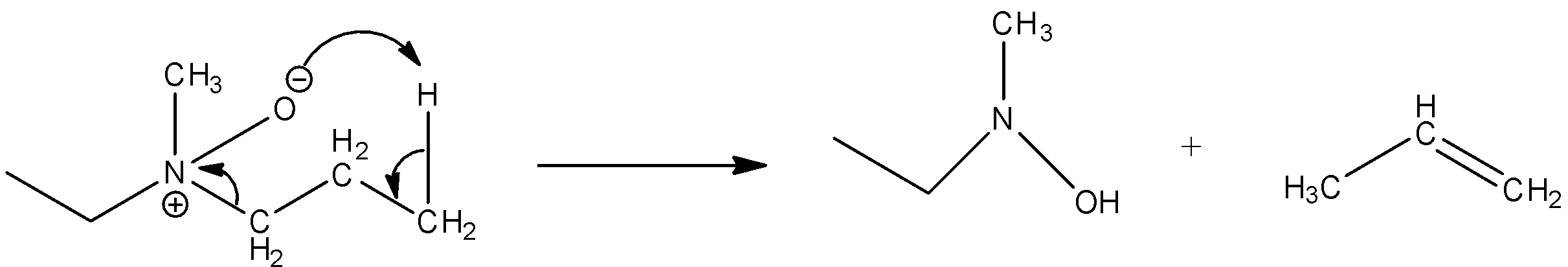
So, the product formed in the reaction is propane.
So, the correct answer is Option B.
Note: Always remember one point regarding amine oxide. Amine oxide does not undergo rapid inversion at the nitrogen atom unlike amine.
In oxidation of primary or secondary amine, peroxybenzoic acid or hydrogen peroxide is used. In such reactions, first amine oxide type intermediate forms which rearrange to form azanol or hydroxylamine.
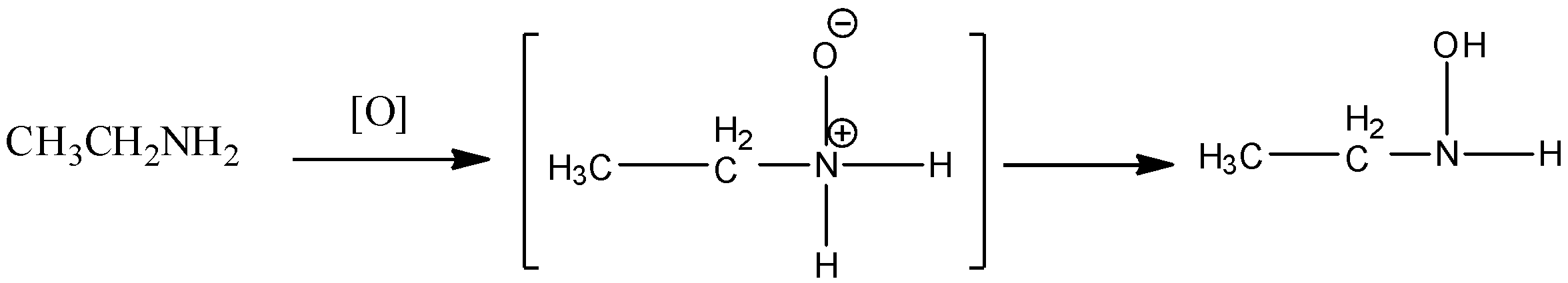
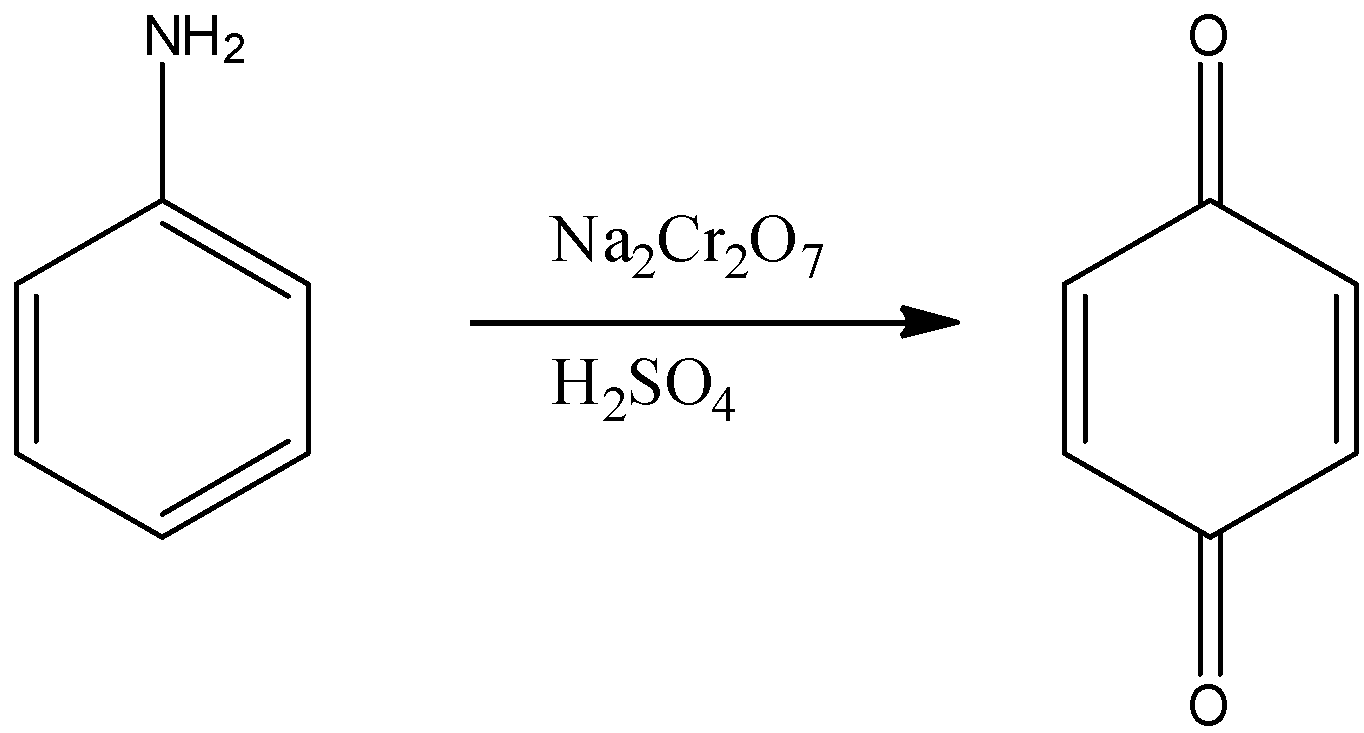
Aromatic primary amines can be oxidized with sodium dichromate in presence of sulphuric acid.