Question
Question: Identify the \[M{g^{2 + }}\] ion, where n and p represent the number of neutrons and protons respect...
Identify the Mg2+ ion, where n and p represent the number of neutrons and protons respectively.
A.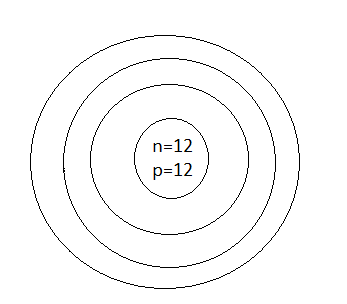
B.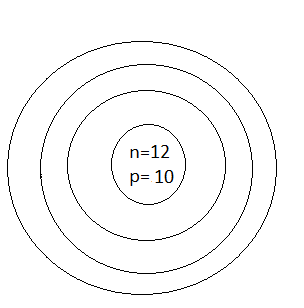
C.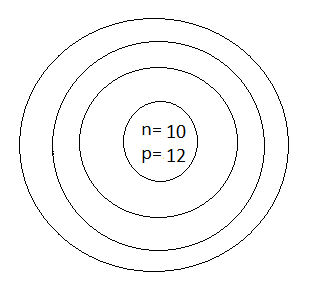
D.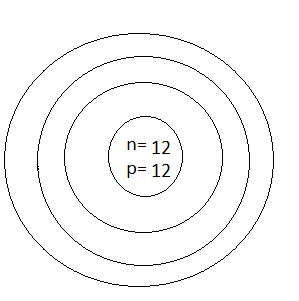
Solution
An atom consists of subatomic particles like electrons, protons, and neutrons. In every neutral atom, the number of protons is equal to the number of electrons. if it is an ion, the electrons will change but not protons. The number of neutrons can be determined by the subtraction of an atomic number from a mass number.
Complete answer:
A chemical element is the purest form of an atom and cannot be broken into simple units by any chemical action. An atom is the smallest particle that cannot be visible to a human eye.
When a neutral atom loses or gains electrons, it becomes an ion.
Given ion is Mg2+ , magnesium is the element with atomic number 12 , which is equal to the number of protons. So, the number of electrons will also be 12 .
The atomic number is nothing but the number of protons, whereas the atomic mass of magnesium is 24 atomic mass units. Thus, the number of neutrons are 12 in magnesium ions or Mg2+.
Thus, the number of protons which was represented by p is 12 and the number of neutrons which was represented by n is also 12
Thus, option D is the correct one.
Note:
The atomic number represented by Z is equal to the number of protons. As an atom must be stable, it should have an equal number of protons and electrons. Thus, a neutral atom has an equal number of protons and electrons, which is different in the case of ions.
