Question
Question: Identify the IUPAC names of chain isomers of pentene: A. \(2 - \) methyl \( - 2 - \) butene B. \...
Identify the IUPAC names of chain isomers of pentene:
A. 2− methyl −2− butene
B. 2− methyl −2− propane
C. 2,2− dimethyl −2− propane
D. 3− methyl −1− butene
Solution
Chain isomers are compounds with the same molecular formula but different structural formula and orientation in the chain. Thus, to identify the isomers of pentene, first we have to draw the structures of each compound. Then we have to check which ones have five carbon atoms and a double bond. The compounds which satisfy both these conditions will be the answer.
Complete step by step answer:
Pentene is a molecule having five carbon atoms in a straight chain having a double bond at one of the ends. Thus, its isomers must possess a total of five carbon atoms and a double bond.
Let us first look at the structure of the first compound given to us, that is 2− methyl −2− butene :
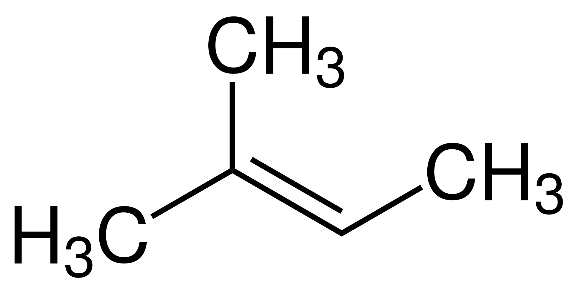
As we can see from the structure, this compound contains five carbon atoms, and also has a double bond. Hence, this is an isomer of pentene.
Now let us examine the structure of the second compound, that is 2− methyl −2− propane :
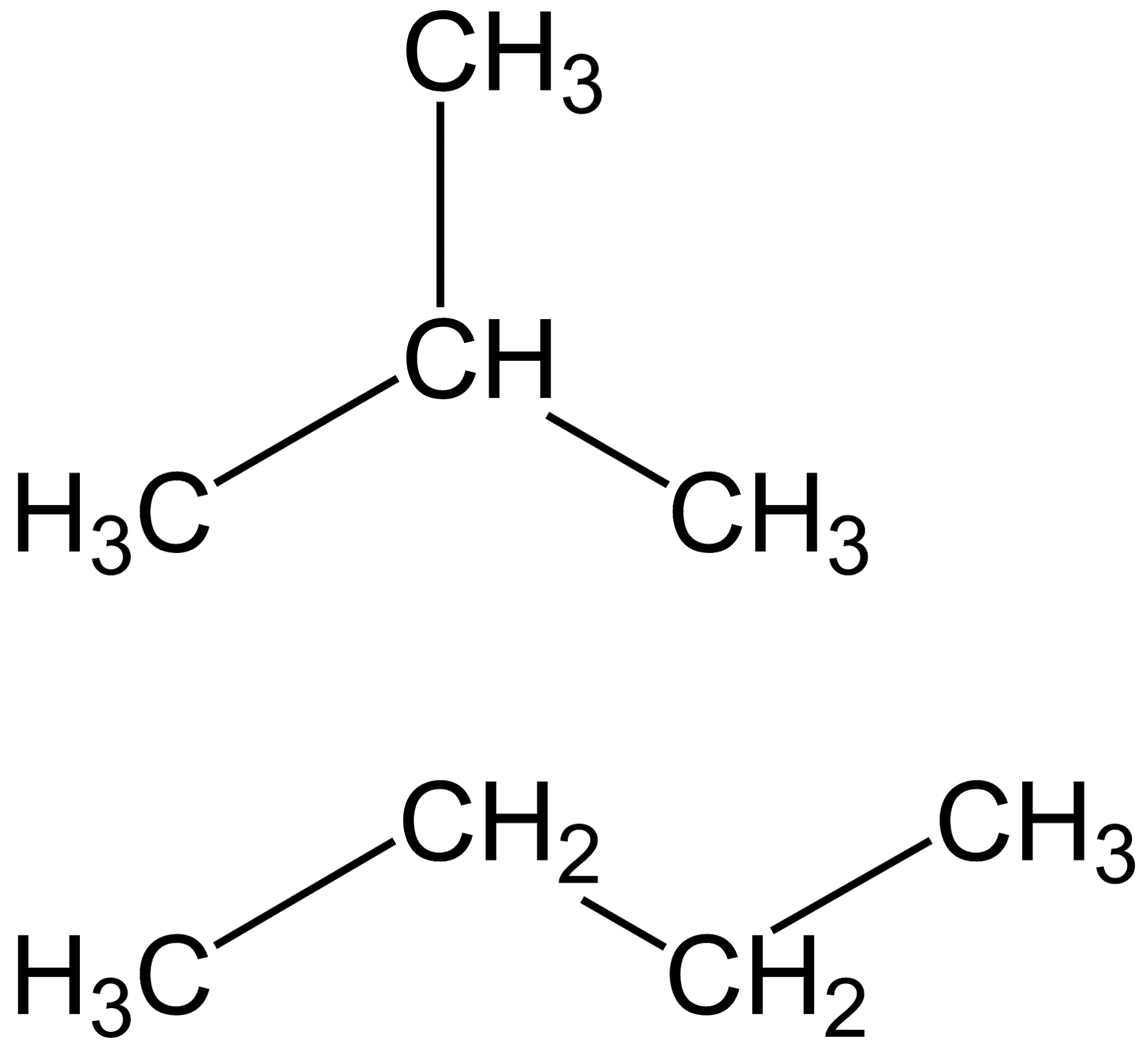
This compound only has four carbon atoms and also, it doesn’t have a double bond. Hence, it is not an isomer of pentene.
Moving on to the third compound, we have 2,2− dimethyl −2− propane :
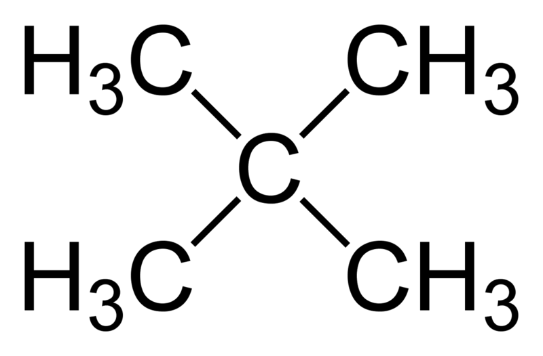
Although this compound has five carbon atoms, it does not have a double bond. Hence, it is not an isomer of pentene.
And the last compound given to us is 3− methyl −1− butene :
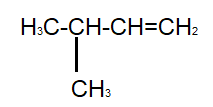
As we can see, this compound satisfies both our conditions, that is, it has five carbon atoms as well as a double bond. Therefore, it is an isomer of pentene.
Hence, we have seen that the isomers of pentene are 2− methyl −2− butene and 3− methyl −1− butene. Thus, the correct options to be marked are A and D.
So, the correct answer is Option A,D.
Note: The second compound, 2− methyl −2− propane is an isomer of butane, and is also known as isobutane. The third compound, 2,2− dimethyl −2− propane is an isomer of pentane, and is known as neopentane.
Note that apart from chain isomerism, the other types of structural isomerism include positional and functional isomerism, metamerism, tautomerism etc.
