Question
Question: Identify the incorrect statement related to \(\text{PC}{{\text{l}}_{5}}\) from the following: A. T...
Identify the incorrect statement related to PCl5 from the following:
A. Three equatorial P−Cl bonds make an angle of 1200 with each other.
B. Two axial P−Cl bonds make an angle of 1800 with each other.
C. Axial P−Cl bonds are longer than equatorial P−Cl bonds.
D. PCl5 molecules is non-reactive
Solution
According to VSEPR theory, gaseous and molten PCl5 is a neutral molecule with trigonal bi-pyramidal as its geometry. This trigonal bi-pyramidal structure persists in nonpolar solvents like CCl4. Look at the structure of PCl5 and the location of the atoms in the molecule to find out the answer.
Complete step by step answer:
PCl5 is a pentavalent compound of phosphorus connected to five chloride ions. In this three chloride ions are in one plane and two left chloride ions are in the perpendicular plane, one above and one being placed below. The structure of PCl5 is
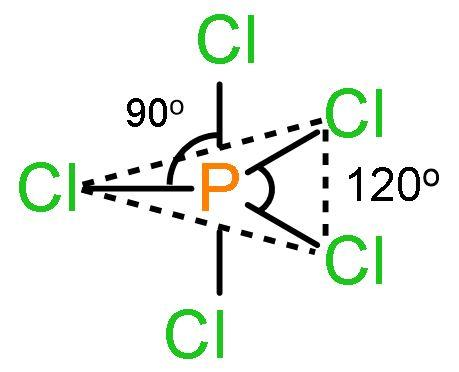
The features of this structure are:
- The three chloride ions in one plane forms a triangle forming central angle of 120o with the central atom phosphorus means the Cl−P−Cl bond is 120o. These chlorine atoms are said to be placed in the equatorial region.
- The two chlorine atoms placed above the plane and another below the plane forms an angle of 90o with the phosphorus atom. The chlorine atoms are said to be placed in the axial region. So, the angle between the chlorine atoms which are in the perpendicular plane is 180o taking phosphorus as the centre of measure. So, ∠(Cl−P−Cl) is 180o.
- The axial bonds are longer than equatorial bonds because the bond pair-bond pair repulsions occur between them due to the presence of lone pairs and angle difference is just 90o. These repulsions make the axial bond pairs to extend their bond length to suffer less repulsion. This makes PCl5 highly reactive which breaks into PCl5→PCl3+Cl2. These axial chlorine atoms are removed as chlorine gas. The repulsion between equatorial bond pairs is negligible as the bond angle is long enough (120o).
The correct answer of this question is option ‘d’ that PCl5 molecules is non-reactive. So, the correct answer is “Option D”.
Note: In the solid state, PCl5 exists in ionic form like [PCl4]+[PCl6]−. The cation [PCl4]+ has the tetrahedral geometry and anion [PCl6]− has octahedral geometry. It was thought that PCl5 also forms a dimeric structure P2Cl10 , but this is not true.
