Question
Question: Identify the functional group in the given compound. 
Solution
You should know that; functional groups are the groups of atoms that are generally attached to the carbon backbone of an organic molecule. So, here find out the carbon chain and then figure out what group is attached to the chain.
Complete step by step solution:
Given that,
A compound with the structure is shown below:
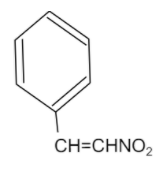
First of all, let us simplify the structure by formulating the chemical formula.
So, the following structure will have the chemical formula as C6H5CH=CHNO2.
We can see that the chemical formula has two functional groups, which consists of a benzene ring and a nitro group. Thus, the structure has three carbons and when naming this chain further, the name will have “prop”. As, there is a presence of double bond, so the name will have “-ene” in it. Now, coming to position of the functional groups, the nitro group i.e. −NO2 is present on the first carbon while the benzene ring is present on the third carbon of the chain. So, during naming of the structure, the nitro group will have greater affinity, so the carbon attached to it will be considered as the first carbon.
And, therefore the IUPAC name of this structure will be 1−nitro,3−benzene,prop−2−ene.
Hence, the structure given in the question will have two functional groups, that are benzene and nitro groups.
Note: It is important to note that the nitro group attached to the chain will have greater affinity while naming as it is electron dense in nature when compared with the benzene group. Benzene, due electron delocalisation will be considered as an electron deficient.
