Question
Question: Identify the functional group in the given compound. 
Solution
We know that functional groups are groups of atoms that decide the chemical reactivity of the compound in which they present.
Complete step by step answer:
We know that there are many functional groups, such as alkane, alkene, alkyne, alcohol, aldehyde, ketone, carboxylic acid etc.
Alkene is the functional group in which double bonds between atoms are present.
Alcohol is a functional group which is bonded to alkyl or aryl chains. The structure of the alcohol functional group is R−OH.
Aldehyde is the functional group in which one alkyl group is bonded to the carbonyl atom. The structure of the aldehyde functional group is –CHO.
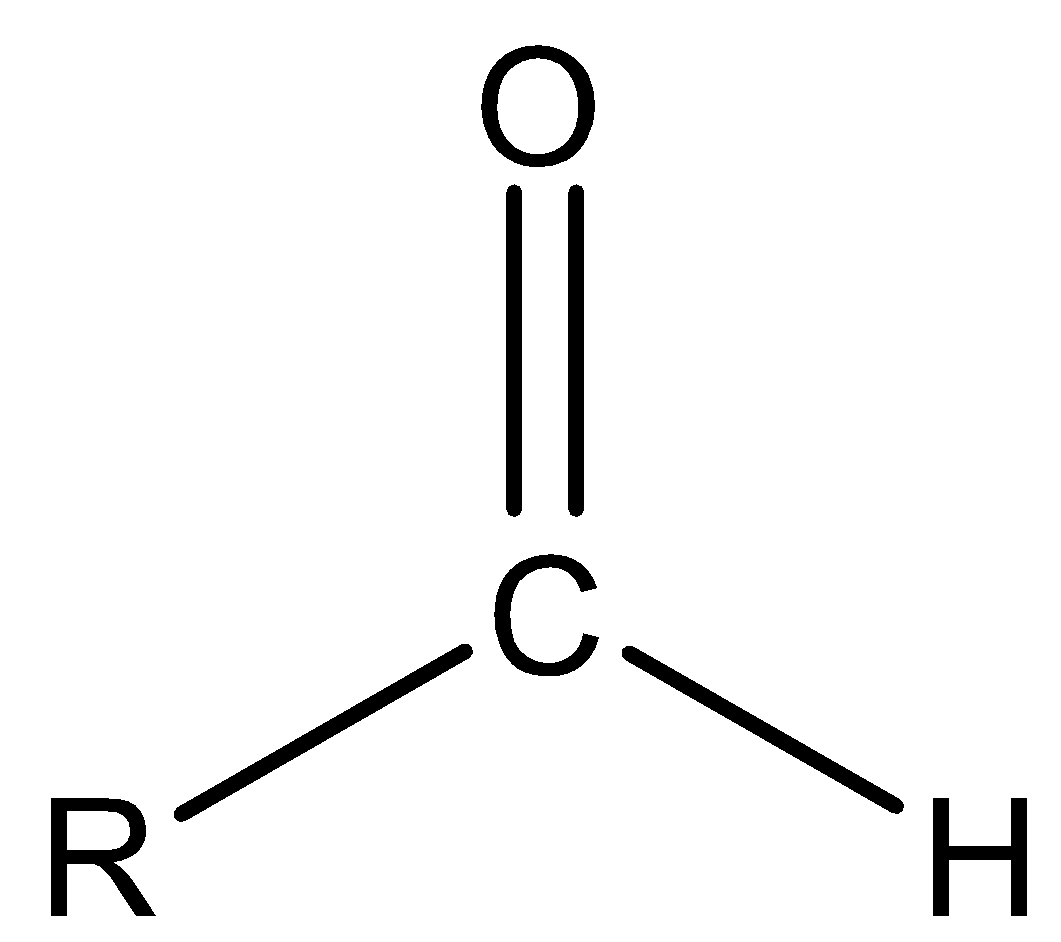
Ether is a functional group in which two alkyl groups are bonded to oxygen atoms and the structure of the ether functional group is R-O-R.
Now, we have to observe the given compound to identify the functional group.
The compound is,
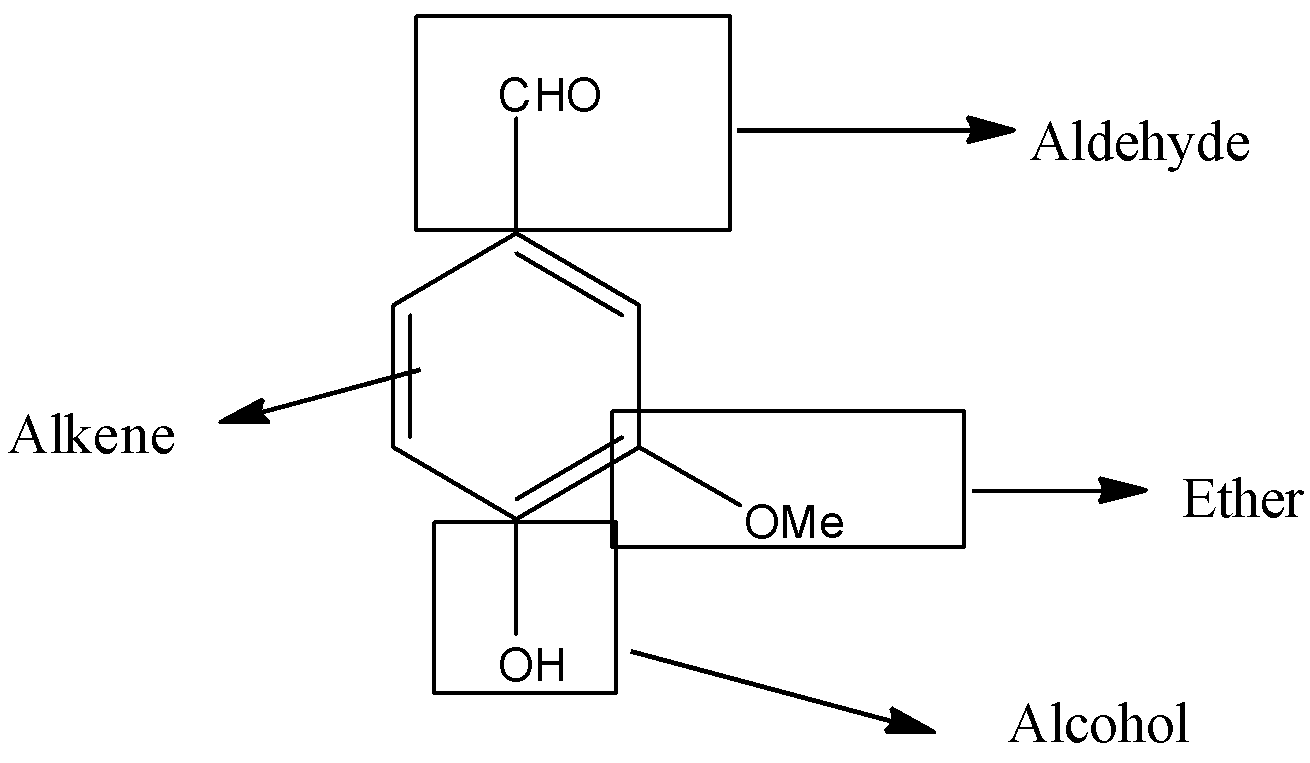
So, we clearly see that in the above compound four functional groups are present. They are, aldehyde, alcohol, ether and alkene.
Note:
The structures of some other functional groups:
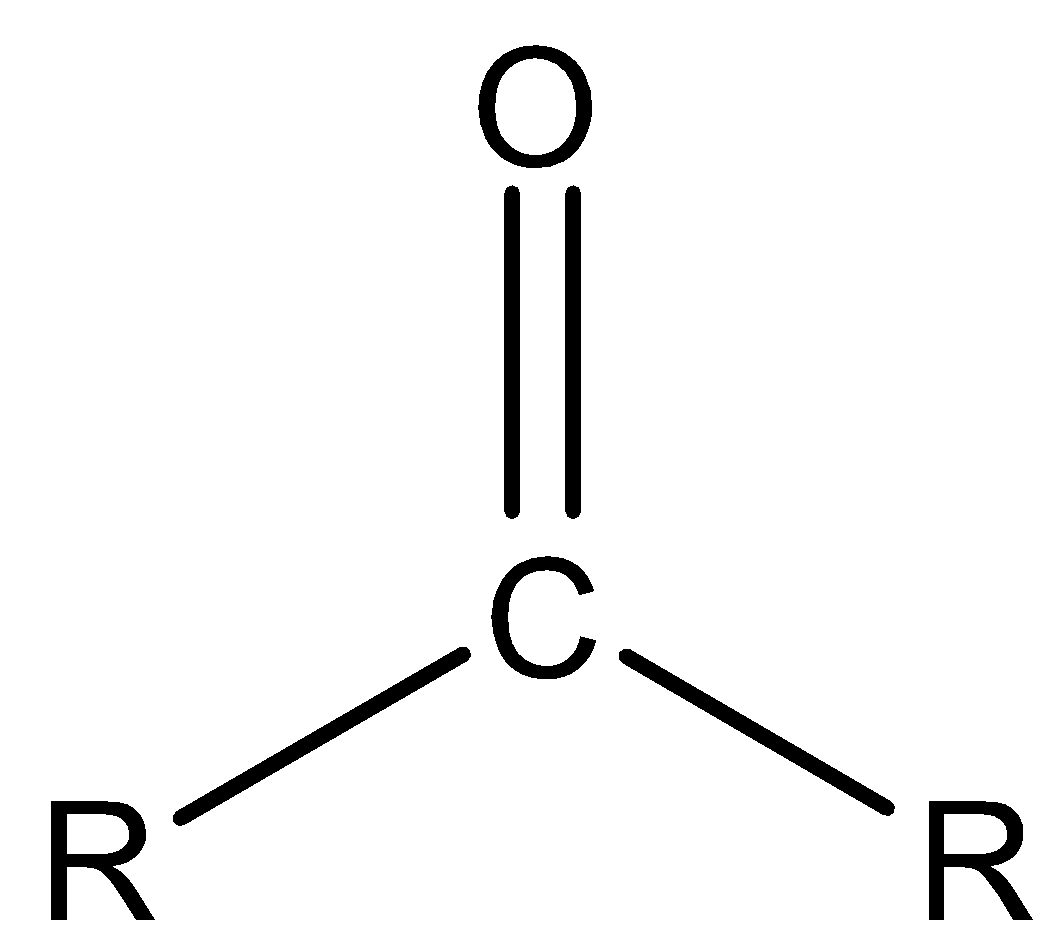
Ketone is a functional group in which two alkyl groups are bonded to the carbonyl group.
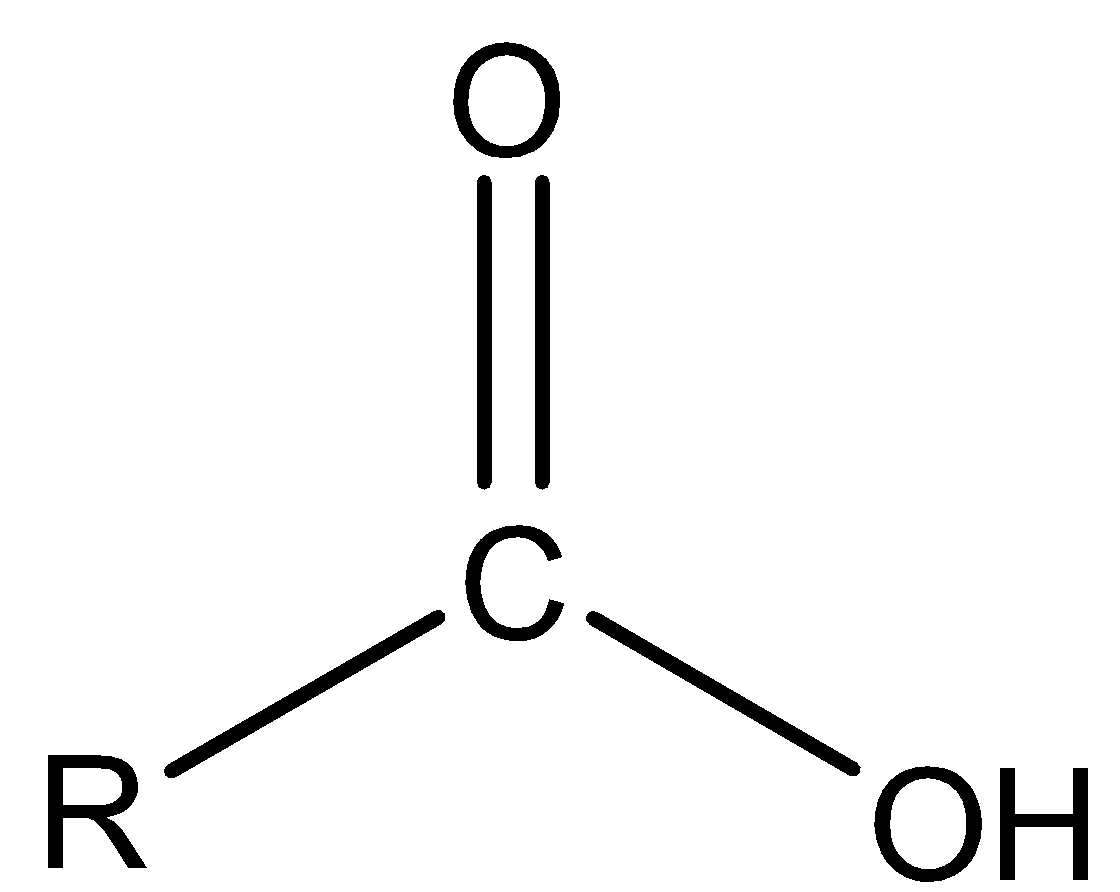
Carboxylic acid is a functional group in which one alkyl group and one alcohol group is bonded to the carbonyl group.
Ester is a functional group in which an alkyl group and OR group is bonded to a carbonyl group.
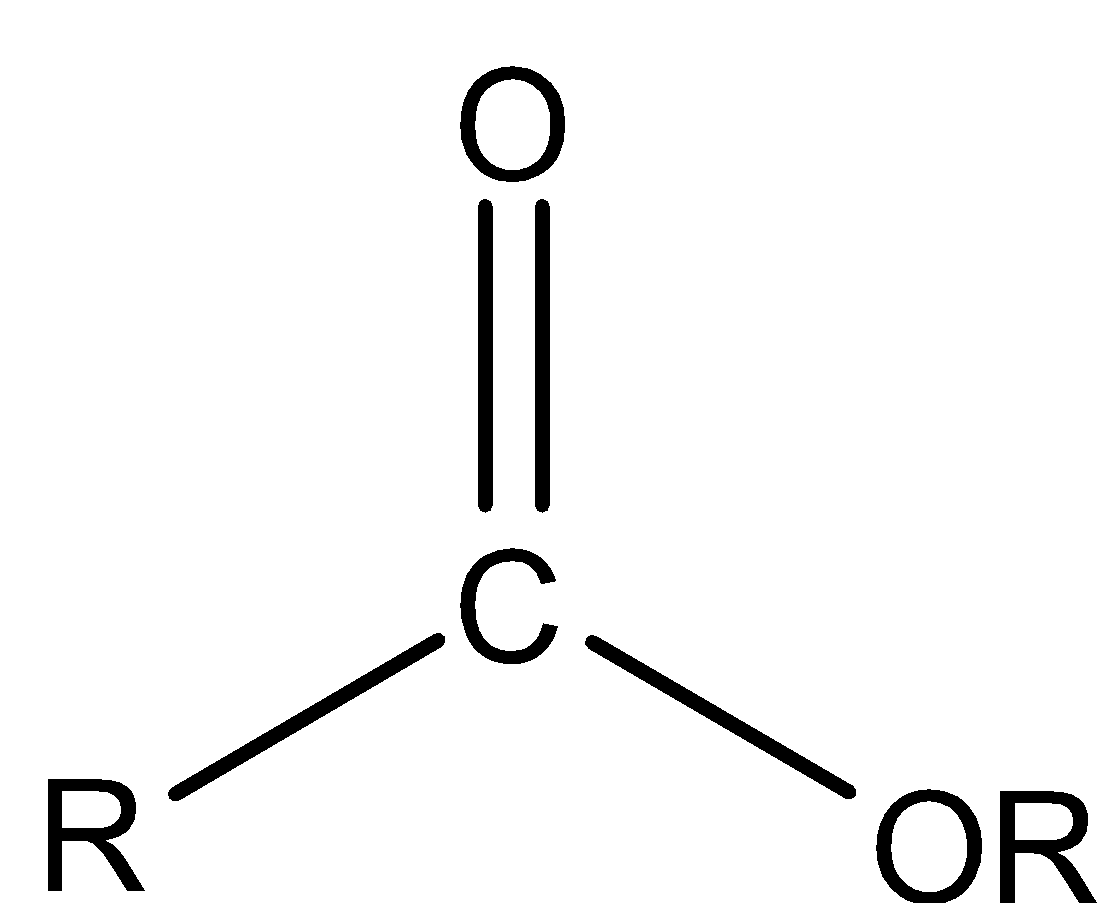
In all the above structures, R represents alkyl groups.
To identify the functional groups from a compound, we must know the structure of all functional groups. In the given compound, four functional groups namely, aldehyde, alcohol, ether and alkene are present.
